| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230755 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 13 Pages |
•The vibrational assignments have been carried for four ring structured molecule.•Stability of the molecule and charge delocalization analyzed using NBO analysis.•The most stable geometry of the compound is determined from PES.•Density plots of HOMO–LUMO energy surface plotted to identify donor and acceptor.•ESP surface plotted to get electrophilic and nucleophilic sites of the molecule.
The Fourier transform (FT-IR) spectrum of Levofloxacin was recorded in the region 4000–400 cm−1 and a complete vibrational assignment of fundamental vibrational modes of the molecule was carried out using density functional method. The observed fundamental modes have been compared with the harmonic vibrational frequencies computed using DFT (B3LYP) method by employing 6-31 G (d, p) basis sets. The most stable geometry of the molecule under investigation has been determined from the potential energy scan. The first-order hyperpolarizability (βo) and other related properties (μ, αo) of Levofloxacin are calculated using density functional theory (DFT) on a finite field approach. UV–vis spectrum of the molecule was recorded and the electronic properties, such as HOMO and LUMO energies were performed by DFT using 6-31 G (d, p) basis sets. Stability of the molecule arising from hyperconjugative interactions, charge delocalization have been analyzed using natural bond orbital analysis (NBO). The calculated HOMO and LUMO energies show that, the charge transfer occurs within the molecule. The other molecular properties like molecular electrostatic potential (MESP), Mulliken population analysis and thermodynamic properties of the title molecule have been calculated.
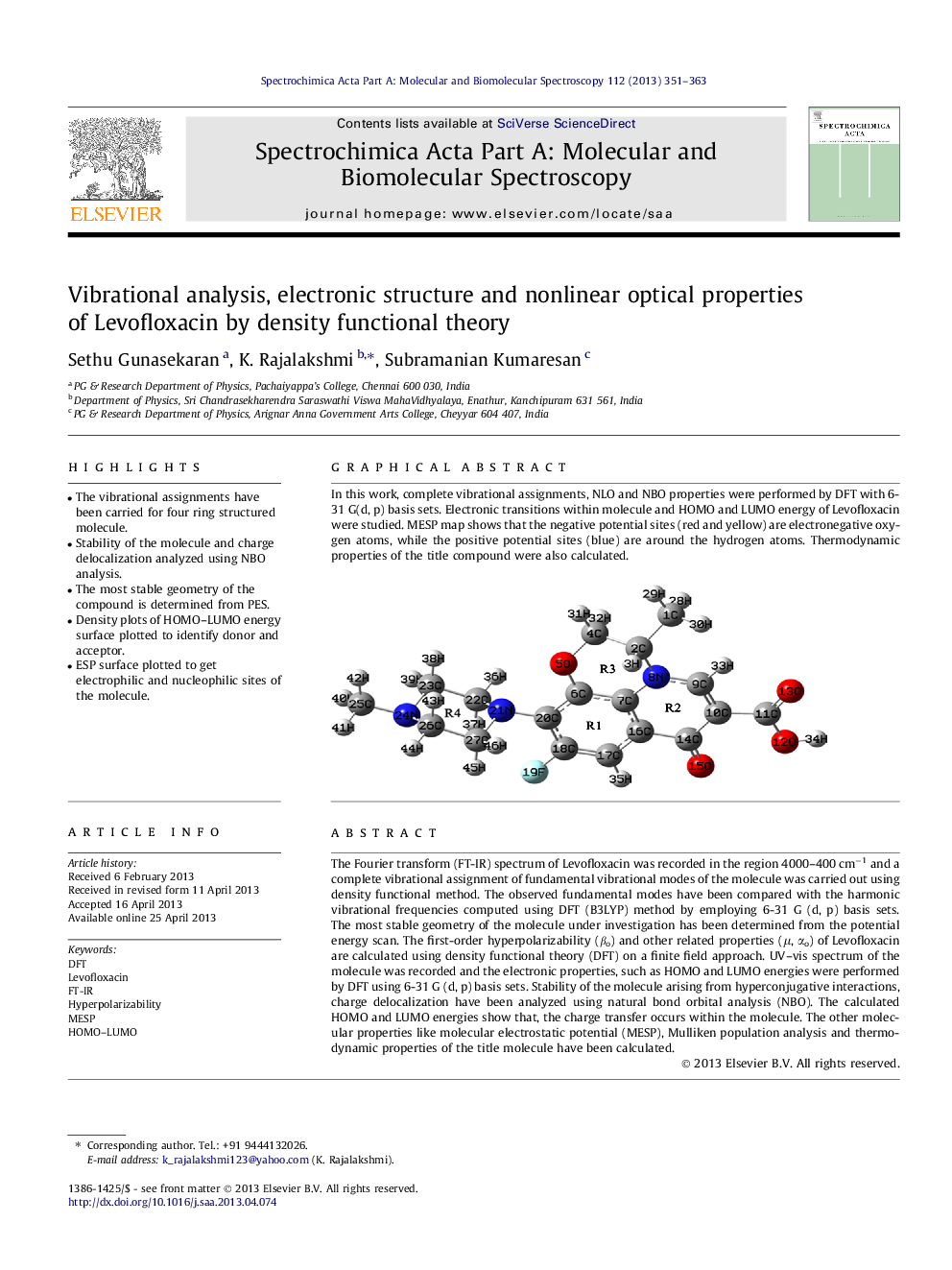
Graphical abstractIn this work, complete vibrational assignments, NLO and NBO properties were performed by DFT with 6-31 G(d, p) basis sets. Electronic transitions within molecule and HOMO and LUMO energy of Levofloxacin were studied. MESP map shows that the negative potential sites (red and yellow) are electronegative oxygen atoms, while the positive potential sites (blue) are around the hydrogen atoms. Thermodynamic properties of the title compound were also calculated.Figure optionsDownload full-size imageDownload as PowerPoint slide