| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230764 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 7 Pages |
•Manuscript deals an easier method for the synthesis of mixed-valence iron complexes.•These are oxo-centered, carboxylate-bridged, trinuclear complexes.•Each iron in the complexes has an octahedral environment.•Mössbauer studies indicated the valence-localized type of complexes.
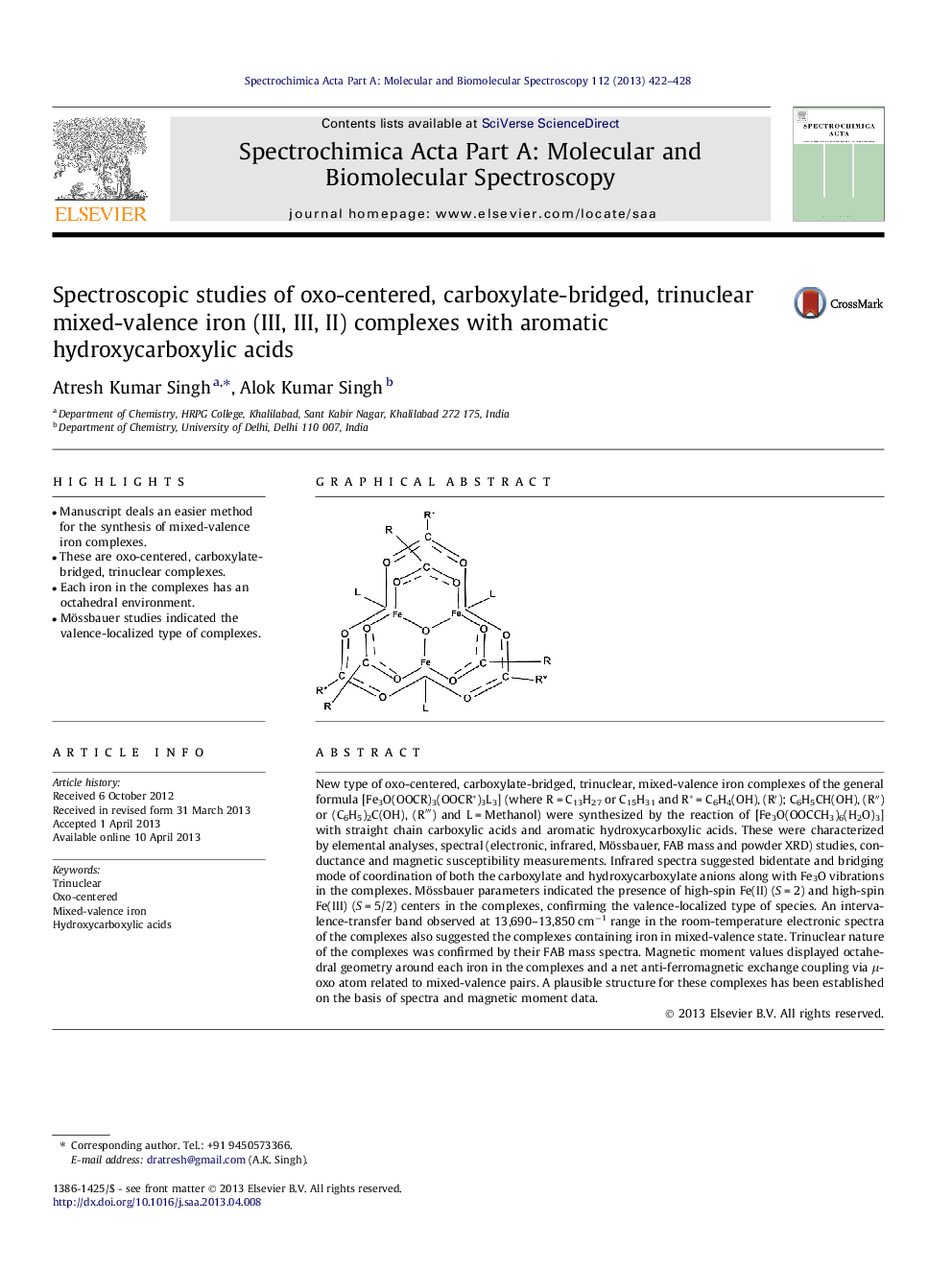
New type of oxo-centered, carboxylate-bridged, trinuclear, mixed-valence iron complexes of the general formula [Fe3O(OOCR)3(OOCR*)3L3] (where R = C13H27 or C15H31 and R* = C6H4(OH), (R′); C6H5CH(OH), (R″) or (C6H5)2C(OH), (R‴R‴) and L = Methanol) were synthesized by the reaction of [Fe3O(OOCCH3)6(H2O)3] with straight chain carboxylic acids and aromatic hydroxycarboxylic acids. These were characterized by elemental analyses, spectral (electronic, infrared, Mössbauer, FAB mass and powder XRD) studies, conductance and magnetic susceptibility measurements. Infrared spectra suggested bidentate and bridging mode of coordination of both the carboxylate and hydroxycarboxylate anions along with Fe3O vibrations in the complexes. Mössbauer parameters indicated the presence of high-spin Fe(ІІ) (S = 2) and high-spin Fe(ІІІ) (S = 5/2) centers in the complexes, confirming the valence-localized type of species. An intervalence-transfer band observed at 13,690–13,850 cm−1 range in the room-temperature electronic spectra of the complexes also suggested the complexes containing iron in mixed-valence state. Trinuclear nature of the complexes was confirmed by their FAB mass spectra. Magnetic moment values displayed octahedral geometry around each iron in the complexes and a net anti-ferromagnetic exchange coupling via μ-oxo atom related to mixed-valence pairs. A plausible structure for these complexes has been established on the basis of spectra and magnetic moment data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide