| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230818 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•FTIR spectra, the stretching of band appearing at (666 cm−1) confirms the formation of rod shaped ZnO particles.•Presence of functional groups and the chemical bonding with Ni confirmed by FTIR.•The average crystalline size is reduced by Ni-doped ZnO.•Optical properties which are appreciable for the fabrication of nano-optoelectronic device.
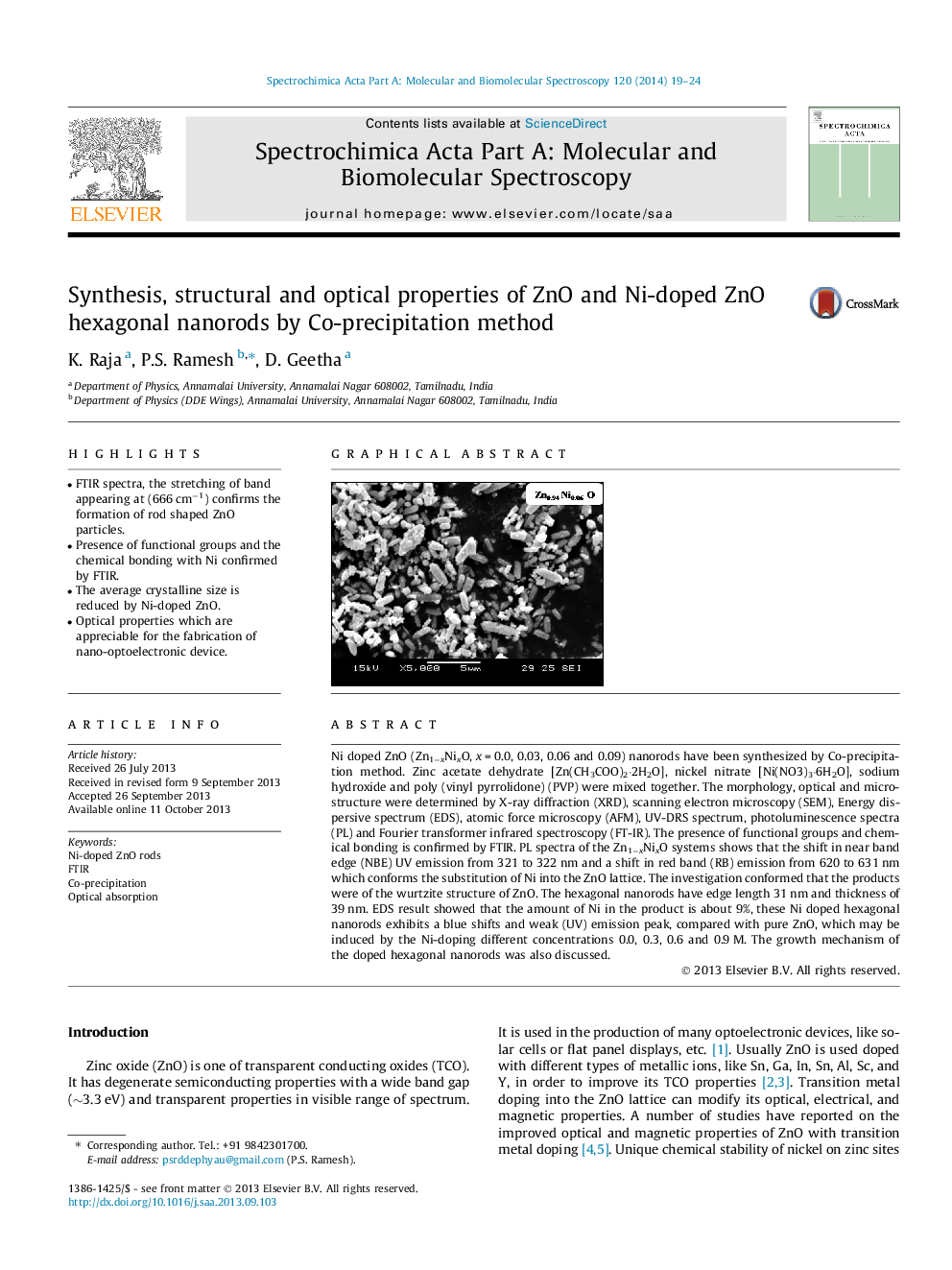
Ni doped ZnO (Zn1−xNixO, x = 0.0, 0.03, 0.06 and 0.09) nanorods have been synthesized by Co-precipitation method. Zinc acetate dehydrate [Zn(CH3COO)2⋅2H2O], nickel nitrate [Ni(NO3)3⋅6H2O], sodium hydroxide and poly (vinyl pyrrolidone) (PVP) were mixed together. The morphology, optical and microstructure were determined by X-ray diffraction (XRD), scanning electron microscopy (SEM), Energy dispersive spectrum (EDS), atomic force microscopy (AFM), UV-DRS spectrum, photoluminescence spectra (PL) and Fourier transformer infrared spectroscopy (FT-IR). The presence of functional groups and chemical bonding is confirmed by FTIR. PL spectra of the Zn1−xNixO systems shows that the shift in near band edge (NBE) UV emission from 321 to 322 nm and a shift in red band (RB) emission from 620 to 631 nm which conforms the substitution of Ni into the ZnO lattice. The investigation conformed that the products were of the wurtzite structure of ZnO. The hexagonal nanorods have edge length 31 nm and thickness of 39 nm. EDS result showed that the amount of Ni in the product is about 9%, these Ni doped hexagonal nanorods exhibits a blue shifts and weak (UV) emission peak, compared with pure ZnO, which may be induced by the Ni-doping different concentrations 0.0, 0.3, 0.6 and 0.9 M. The growth mechanism of the doped hexagonal nanorods was also discussed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide