| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230836 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 10 Pages |
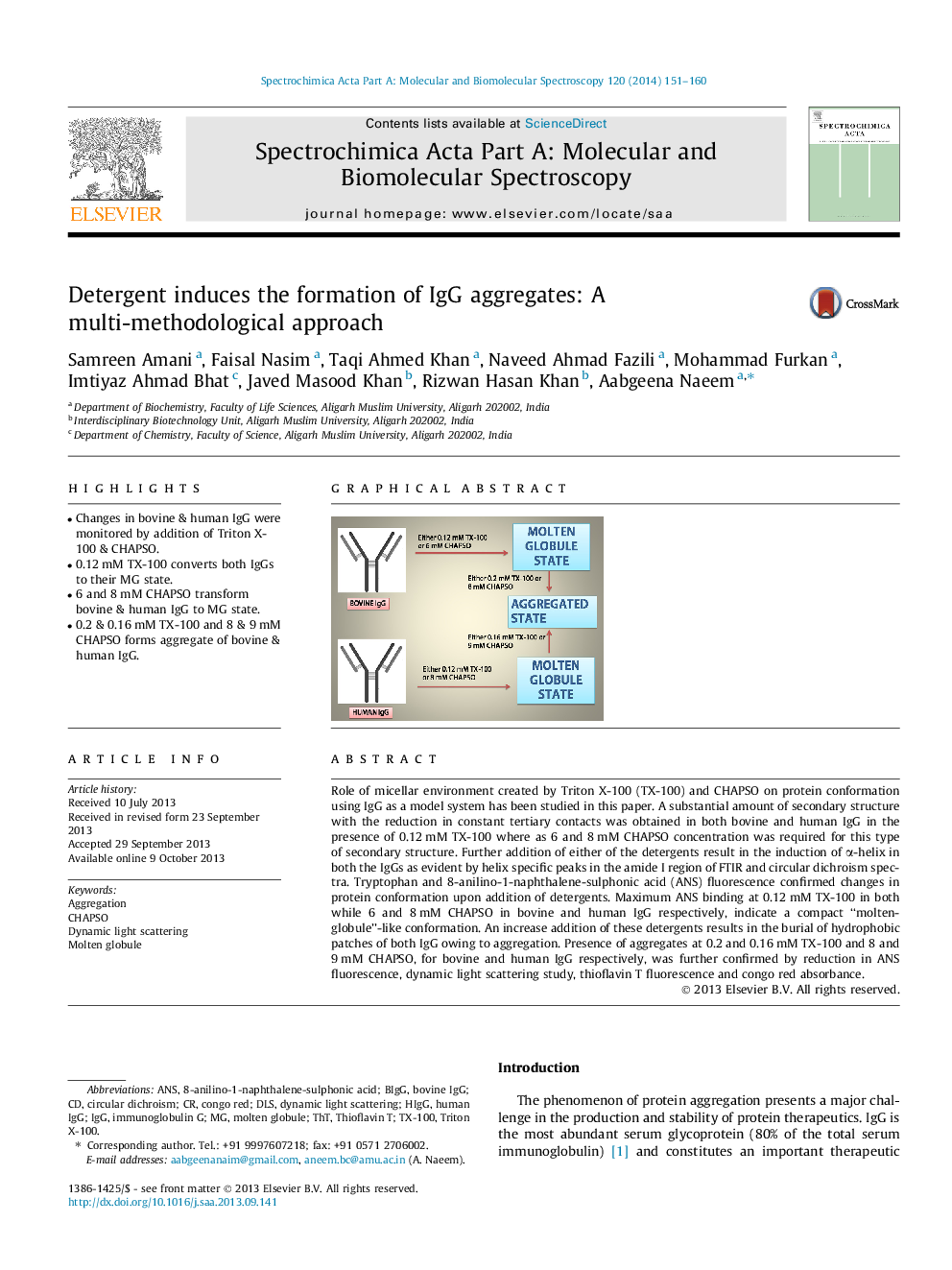
•Changes in bovine & human IgG were monitored by addition of Triton X-100 & CHAPSO.•0.12 mM TX-100 converts both IgGs to their MG state.•6 and 8 mM CHAPSO transform bovine & human IgG to MG state.•0.2 & 0.16 mM TX-100 and 8 & 9 mM CHAPSO forms aggregate of bovine & human IgG.
Role of micellar environment created by Triton X-100 (TX-100) and CHAPSO on protein conformation using IgG as a model system has been studied in this paper. A substantial amount of secondary structure with the reduction in constant tertiary contacts was obtained in both bovine and human IgG in the presence of 0.12 mM TX-100 where as 6 and 8 mM CHAPSO concentration was required for this type of secondary structure. Further addition of either of the detergents result in the induction of α-helix in both the IgGs as evident by helix specific peaks in the amide I region of FTIR and circular dichroism spectra. Tryptophan and 8-anilino-1-naphthalene-sulphonic acid (ANS) fluorescence confirmed changes in protein conformation upon addition of detergents. Maximum ANS binding at 0.12 mM TX-100 in both while 6 and 8 mM CHAPSO in bovine and human IgG respectively, indicate a compact ‘‘molten-globule’’-like conformation. An increase addition of these detergents results in the burial of hydrophobic patches of both IgG owing to aggregation. Presence of aggregates at 0.2 and 0.16 mM TX-100 and 8 and 9 mM CHAPSO, for bovine and human IgG respectively, was further confirmed by reduction in ANS fluorescence, dynamic light scattering study, thioflavin T fluorescence and congo red absorbance.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide