| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230839 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Pyrazolines are attractive drug scaffold with many biological applications.•Synthesis of new pyrazoline derivatives equipped with N-acyl arms and long alkoxy side chains.•Spectral, self-assembly, antifungal and anti-inflammatory studies.•Effect of alkoxy chain length on molecular packing and bioactivity.
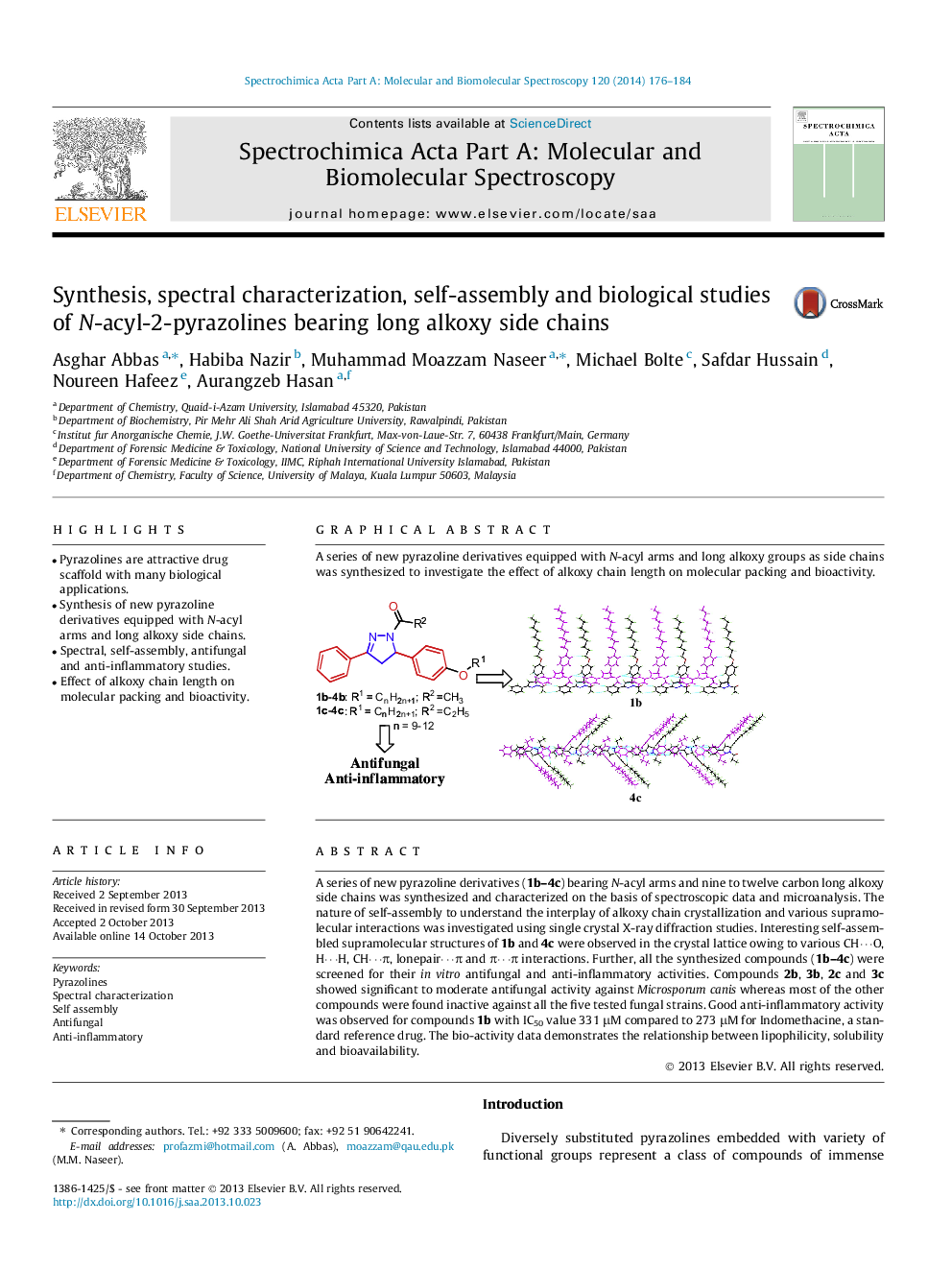
A series of new pyrazoline derivatives (1b–4c) bearing N-acyl arms and nine to twelve carbon long alkoxy side chains was synthesized and characterized on the basis of spectroscopic data and microanalysis. The nature of self-assembly to understand the interplay of alkoxy chain crystallization and various supramolecular interactions was investigated using single crystal X-ray diffraction studies. Interesting self-assembled supramolecular structures of 1b and 4c were observed in the crystal lattice owing to various CH⋯O, H⋯H, CH⋯π, lonepair⋯π and π⋯π interactions. Further, all the synthesized compounds (1b–4c) were screened for their in vitro antifungal and anti-inflammatory activities. Compounds 2b, 3b, 2c and 3c showed significant to moderate antifungal activity against Microsporum canis whereas most of the other compounds were found inactive against all the five tested fungal strains. Good anti-inflammatory activity was observed for compounds 1b with IC50 value 331 μM compared to 273 μM for Indomethacine, a standard reference drug. The bio-activity data demonstrates the relationship between lipophilicity, solubility and bioavailability.
Graphical abstractA series of new pyrazoline derivatives equipped with N-acyl arms and long alkoxy groups as side chains was synthesized to investigate the effect of alkoxy chain length on molecular packing and bioactivity.Figure optionsDownload full-size imageDownload as PowerPoint slide