| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230845 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Three new co-crystals of pyridine-2-carboxamide have been synthesized and characterized.•It is the length of carbon chain of co-crystal formers that control the co-crystal structure.•We investigated detailed the interactions by Hirshfeld surface solid-state vibrational spectroscopy.
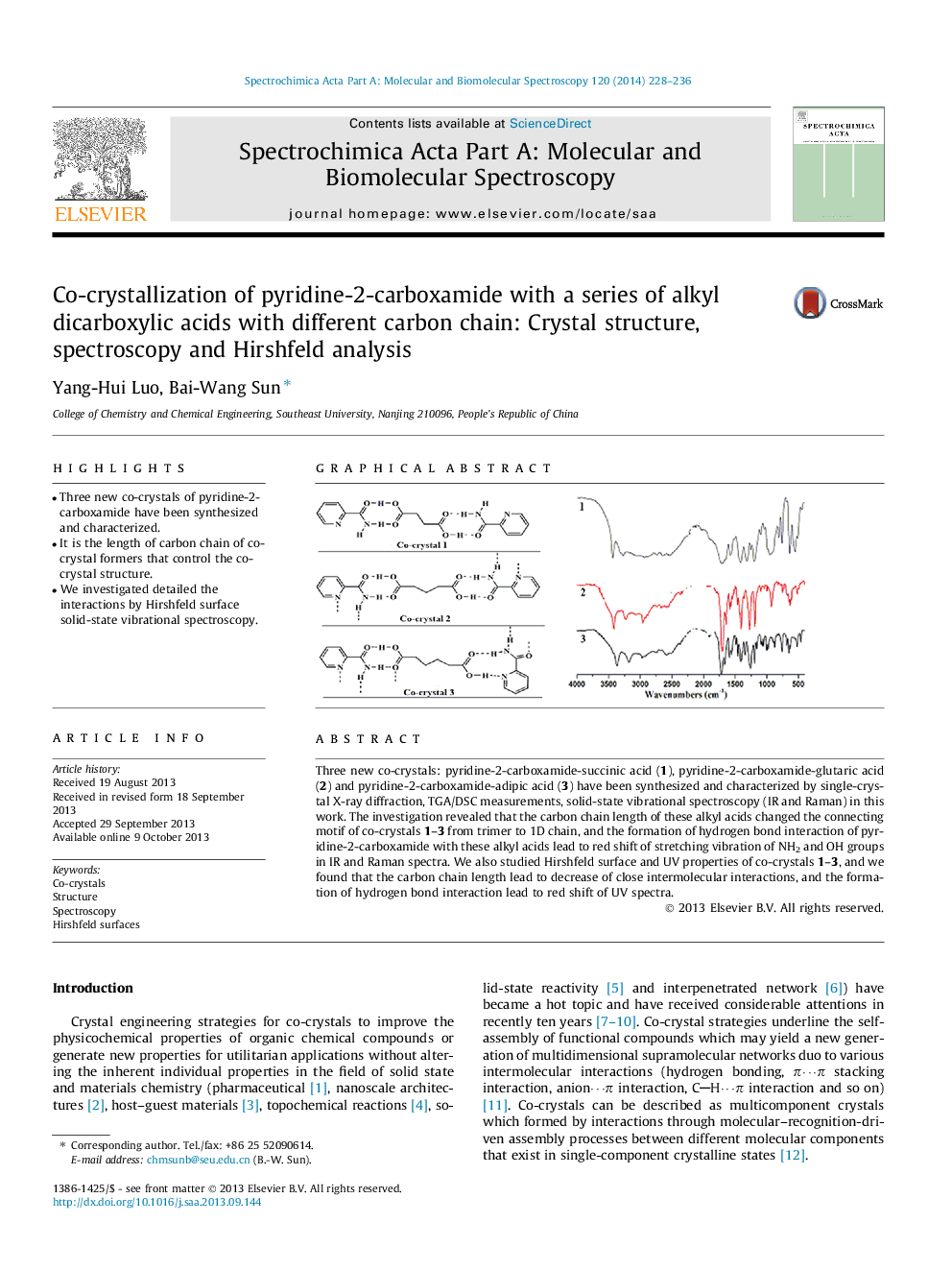
Three new co-crystals: pyridine-2-carboxamide-succinic acid (1), pyridine-2-carboxamide-glutaric acid (2) and pyridine-2-carboxamide-adipic acid (3) have been synthesized and characterized by single-crystal X-ray diffraction, TGA/DSC measurements, solid-state vibrational spectroscopy (IR and Raman) in this work. The investigation revealed that the carbon chain length of these alkyl acids changed the connecting motif of co-crystals 1–3 from trimer to 1D chain, and the formation of hydrogen bond interaction of pyridine-2-carboxamide with these alkyl acids lead to red shift of stretching vibration of NH2 and OH groups in IR and Raman spectra. We also studied Hirshfeld surface and UV properties of co-crystals 1–3, and we found that the carbon chain length lead to decrease of close intermolecular interactions, and the formation of hydrogen bond interaction lead to red shift of UV spectra.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide