| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230863 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•Absorption and emission spectral studies have been carried out.•HOMO–LUMO – Probable charge transfer taking place.•ESIPT process explained in detail.•Competition of intra and intermolecular hydrogen bonding.
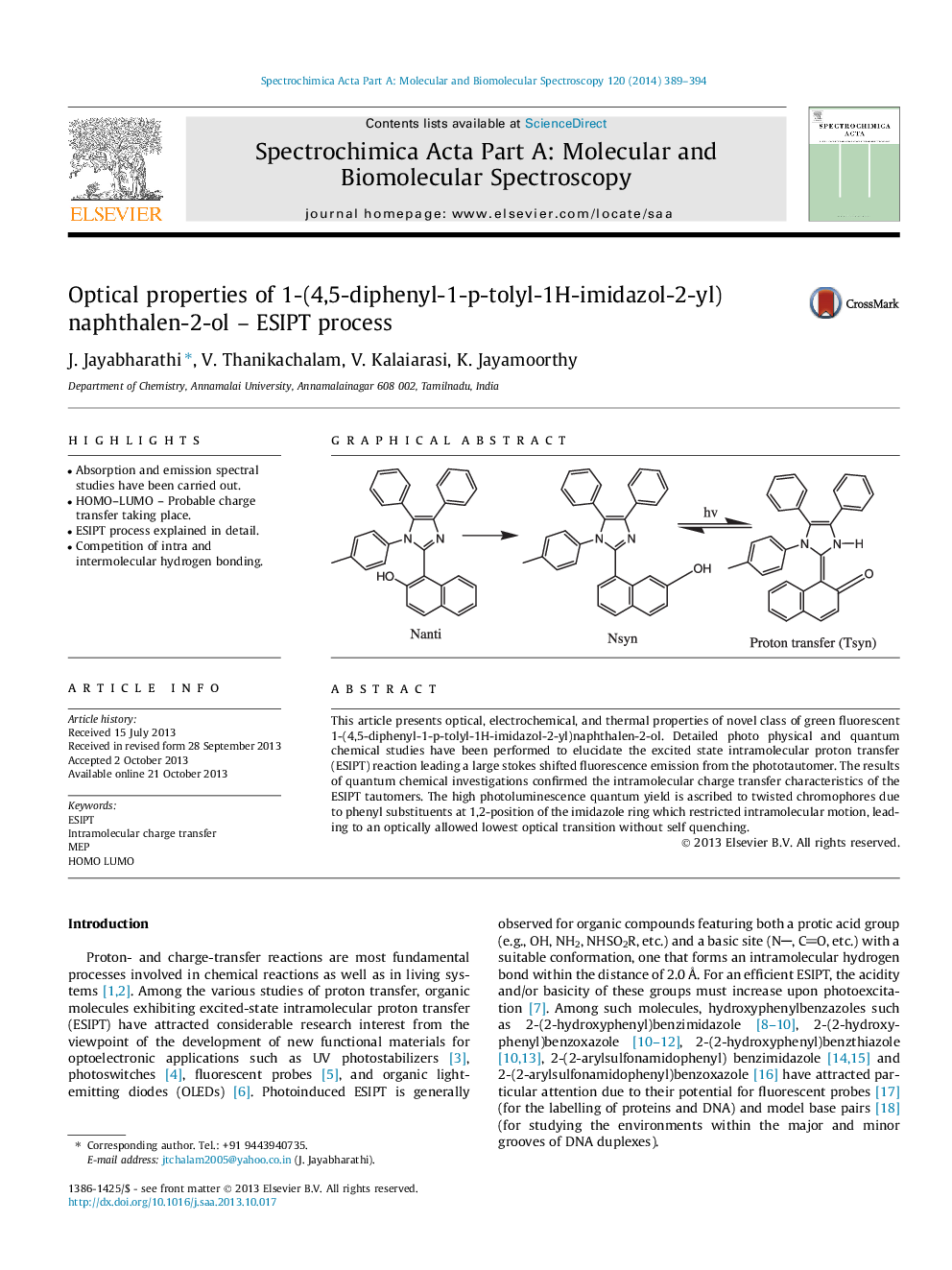
This article presents optical, electrochemical, and thermal properties of novel class of green fluorescent 1-(4,5-diphenyl-1-p-tolyl-1H-imidazol-2-yl)naphthalen-2-ol. Detailed photo physical and quantum chemical studies have been performed to elucidate the excited state intramolecular proton transfer (ESIPT) reaction leading a large stokes shifted fluorescence emission from the phototautomer. The results of quantum chemical investigations confirmed the intramolecular charge transfer characteristics of the ESIPT tautomers. The high photoluminescence quantum yield is ascribed to twisted chromophores due to phenyl substituents at 1,2-position of the imidazole ring which restricted intramolecular motion, leading to an optically allowed lowest optical transition without self quenching.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide