| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230879 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
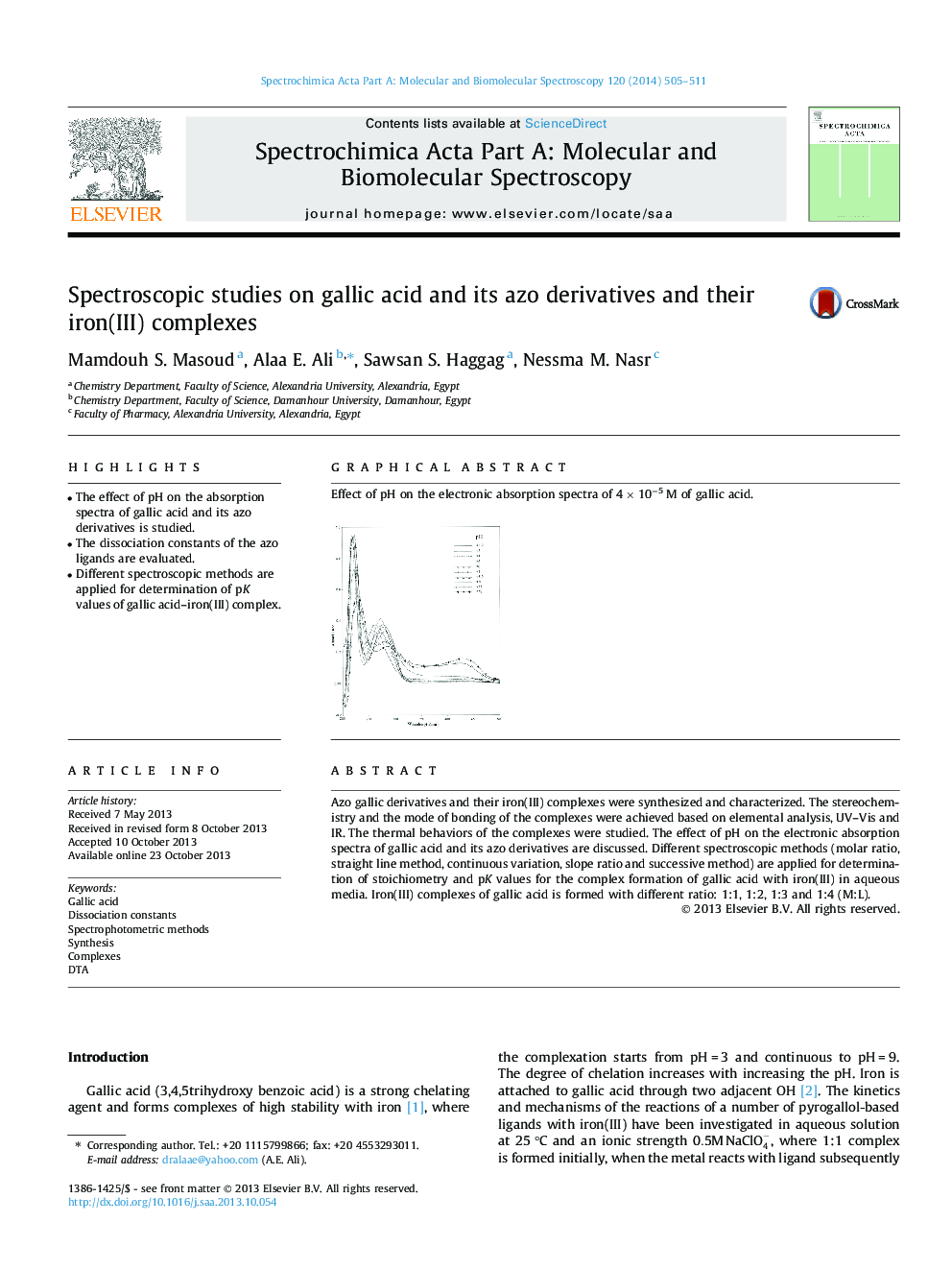
•The effect of pH on the absorption spectra of gallic acid and its azo derivatives is studied.•The dissociation constants of the azo ligands are evaluated.•Different spectroscopic methods are applied for determination of pK values of gallic acid–iron(III) complex.
Azo gallic derivatives and their iron(III) complexes were synthesized and characterized. The stereochemistry and the mode of bonding of the complexes were achieved based on elemental analysis, UV–Vis and IR. The thermal behaviors of the complexes were studied. The effect of pH on the electronic absorption spectra of gallic acid and its azo derivatives are discussed. Different spectroscopic methods (molar ratio, straight line method, continuous variation, slope ratio and successive method) are applied for determination of stoichiometry and pK values for the complex formation of gallic acid with iron(III) in aqueous media. Iron(III) complexes of gallic acid is formed with different ratio: 1:1, 1:2, 1:3 and 1:4 (M:L).
Graphical abstractEffect of pH on the electronic absorption spectra of 4 × 10−5 M of gallic acid.Figure optionsDownload full-size imageDownload as PowerPoint slide