| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230880 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
•The fluorescence quenching of human serum albumin (HSA) by triphenyltin (TPT) is static.•The binding of TPT and HSA is a result of the formation of TPT–HSA complex.•The energy transfer of HSA to TPT occurs.•Hydrophobic force is the main binding forces.•Addition of TPT changes the conformation of HSA.
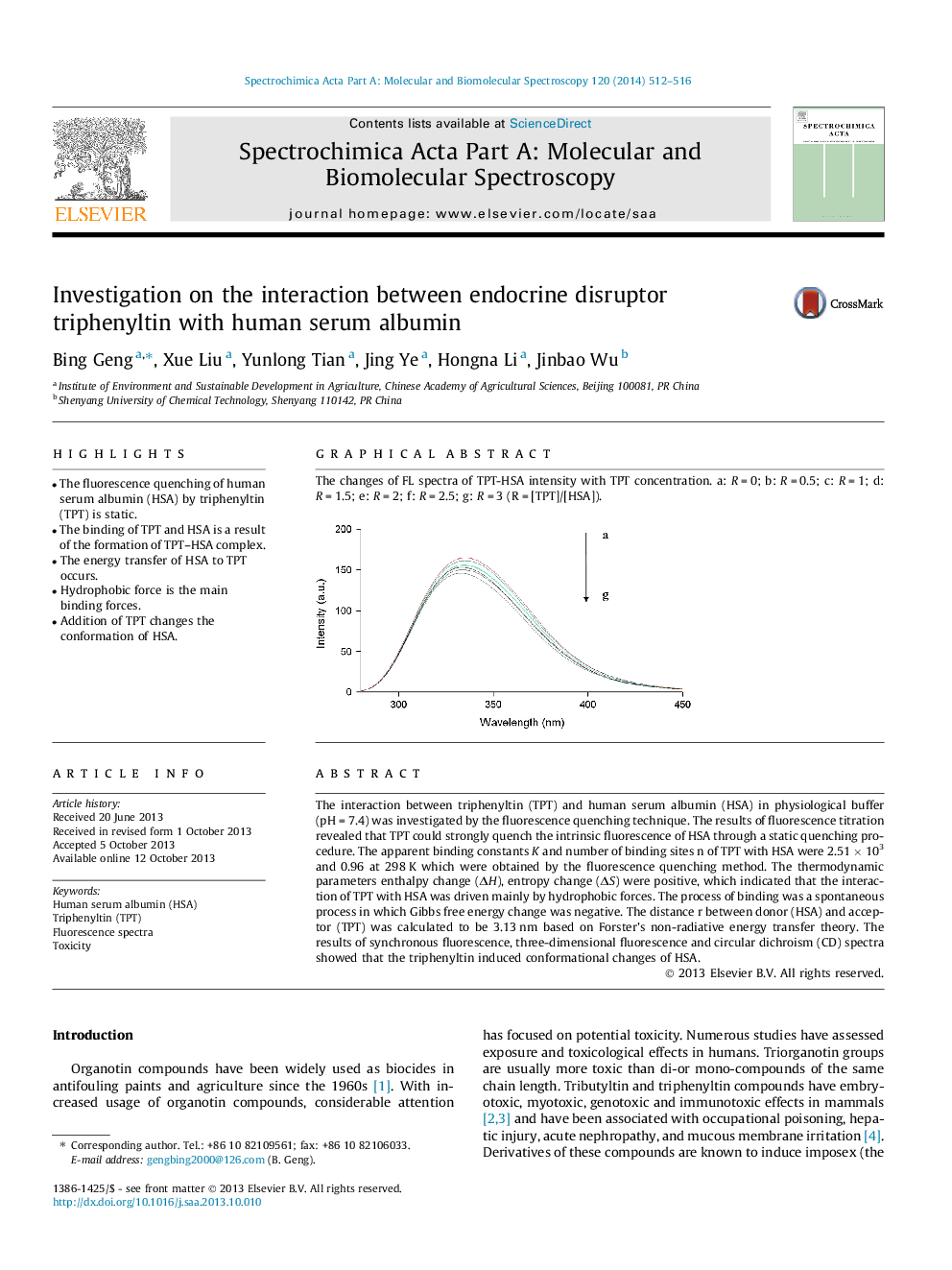
The interaction between triphenyltin (TPT) and human serum albumin (HSA) in physiological buffer (pH = 7.4) was investigated by the fluorescence quenching technique. The results of fluorescence titration revealed that TPT could strongly quench the intrinsic fluorescence of HSA through a static quenching procedure. The apparent binding constants K and number of binding sites n of TPT with HSA were 2.51 × 103 and 0.96 at 298 K which were obtained by the fluorescence quenching method. The thermodynamic parameters enthalpy change (ΔH), entropy change (ΔS) were positive, which indicated that the interaction of TPT with HSA was driven mainly by hydrophobic forces. The process of binding was a spontaneous process in which Gibbs free energy change was negative. The distance r between donor (HSA) and acceptor (TPT) was calculated to be 3.13 nm based on Forster’s non-radiative energy transfer theory. The results of synchronous fluorescence, three-dimensional fluorescence and circular dichroism (CD) spectra showed that the triphenyltin induced conformational changes of HSA.
Graphical abstractThe changes of FL spectra of TPT-HSA intensity with TPT concentration. a: R = 0; b: R = 0.5; c: R = 1; d: R = 1.5; e: R = 2; f: R = 2.5; g: R = 3 (R = [TPT]/[HSA]).Figure optionsDownload full-size imageDownload as PowerPoint slide