| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230966 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
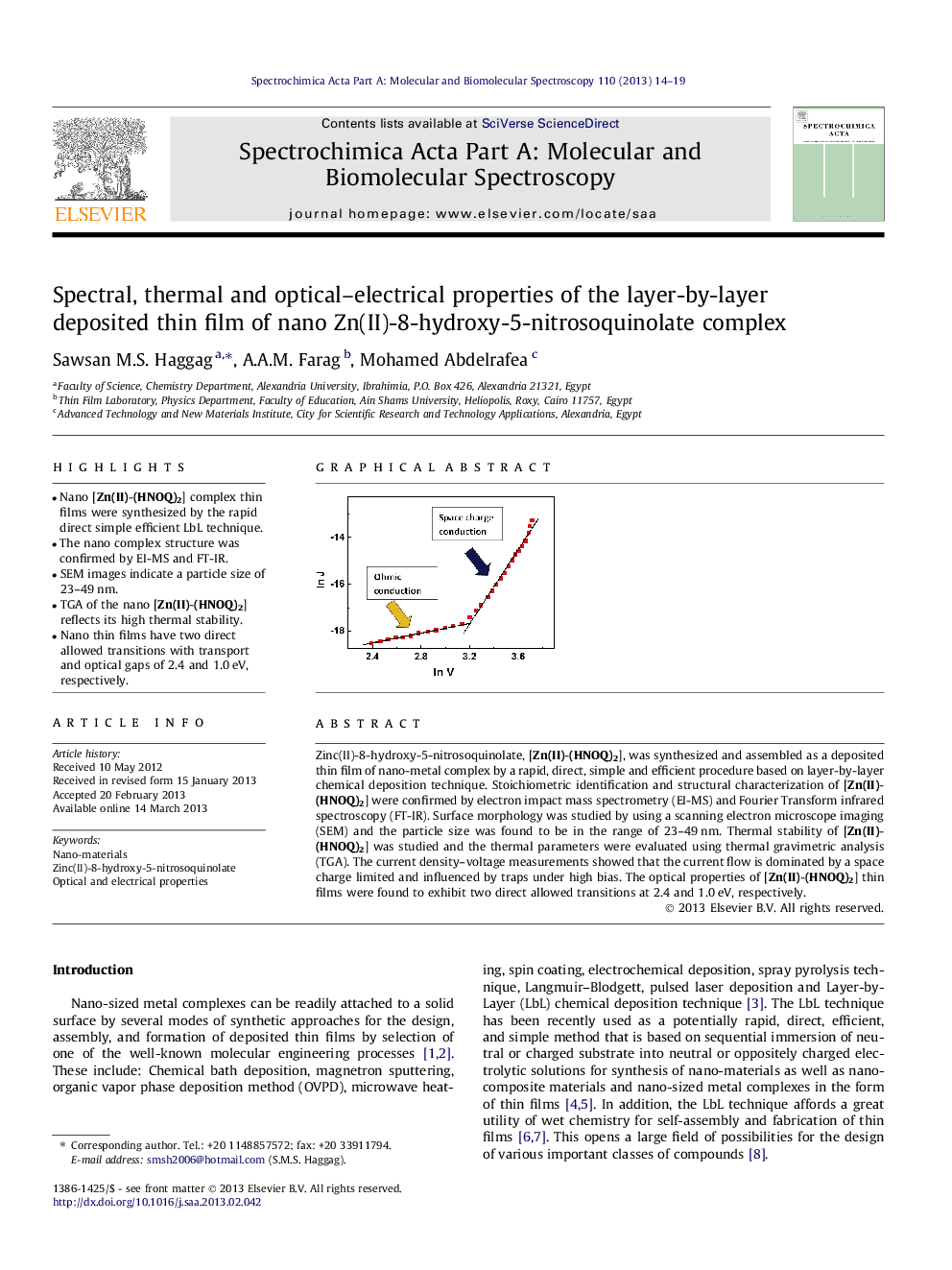
•Nano [Zn(II)-(HNOQ)2] complex thin films were synthesized by the rapid direct simple efficient LbL technique.•The nano complex structure was confirmed by EI-MS and FT-IR.•SEM images indicate a particle size of 23–49 nm.•TGA of the nano [Zn(II)-(HNOQ)2] reflects its high thermal stability.•Nano thin films have two direct allowed transitions with transport and optical gaps of 2.4 and 1.0 eV, respectively.
Zinc(II)-8-hydroxy-5-nitrosoquinolate, [Zn(II)-(HNOQ)2], was synthesized and assembled as a deposited thin film of nano-metal complex by a rapid, direct, simple and efficient procedure based on layer-by-layer chemical deposition technique. Stoichiometric identification and structural characterization of [Zn(II)-(HNOQ)2] were confirmed by electron impact mass spectrometry (EI-MS) and Fourier Transform infrared spectroscopy (FT-IR). Surface morphology was studied by using a scanning electron microscope imaging (SEM) and the particle size was found to be in the range of 23–49 nm. Thermal stability of [Zn(II)-(HNOQ)2] was studied and the thermal parameters were evaluated using thermal gravimetric analysis (TGA). The current density–voltage measurements showed that the current flow is dominated by a space charge limited and influenced by traps under high bias. The optical properties of [Zn(II)-(HNOQ)2] thin films were found to exhibit two direct allowed transitions at 2.4 and 1.0 eV, respectively.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide