| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230979 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
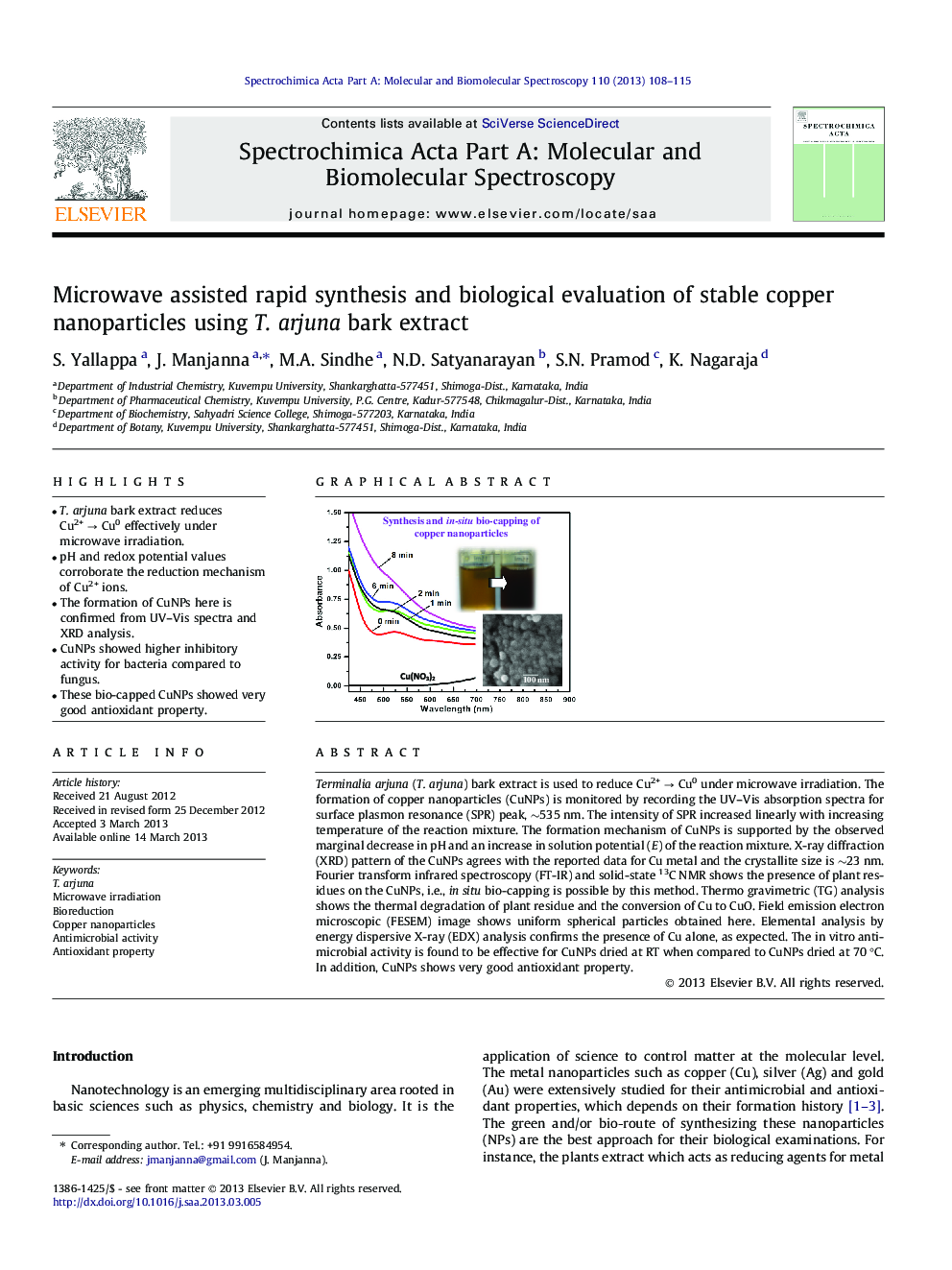
•T. arjuna bark extract reduces Cu2+ → Cu0 effectively under microwave irradiation.•pH and redox potential values corroborate the reduction mechanism of Cu2+ ions.•The formation of CuNPs here is confirmed from UV–Vis spectra and XRD analysis.•CuNPs showed higher inhibitory activity for bacteria compared to fungus.•These bio-capped CuNPs showed very good antioxidant property.
Terminalia arjuna (T. arjuna) bark extract is used to reduce Cu2+ → Cu0 under microwave irradiation. The formation of copper nanoparticles (CuNPs) is monitored by recording the UV–Vis absorption spectra for surface plasmon resonance (SPR) peak, ∼535 nm. The intensity of SPR increased linearly with increasing temperature of the reaction mixture. The formation mechanism of CuNPs is supported by the observed marginal decrease in pH and an increase in solution potential (E) of the reaction mixture. X-ray diffraction (XRD) pattern of the CuNPs agrees with the reported data for Cu metal and the crystallite size is ∼23 nm. Fourier transform infrared spectroscopy (FT-IR) and solid-state 13C NMR shows the presence of plant residues on the CuNPs, i.e., in situ bio-capping is possible by this method. Thermo gravimetric (TG) analysis shows the thermal degradation of plant residue and the conversion of Cu to CuO. Field emission electron microscopic (FESEM) image shows uniform spherical particles obtained here. Elemental analysis by energy dispersive X-ray (EDX) analysis confirms the presence of Cu alone, as expected. The in vitro antimicrobial activity is found to be effective for CuNPs dried at RT when compared to CuNPs dried at 70 °C. In addition, CuNPs shows very good antioxidant property.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide