| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230980 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
•Some 2,5-dimethyl-3-furyl chalcones been synthesized using hydroxyapatite catalyzed aldol condensation.•Better yields of the chalcones (more than 80%) obtained.•These chalcones were characterized by their analytical and spectroscopic data.•Antimicrobial activities of these chalcones have been studied.•Easy-workup synthesis involving shorter reaction time was adopted.
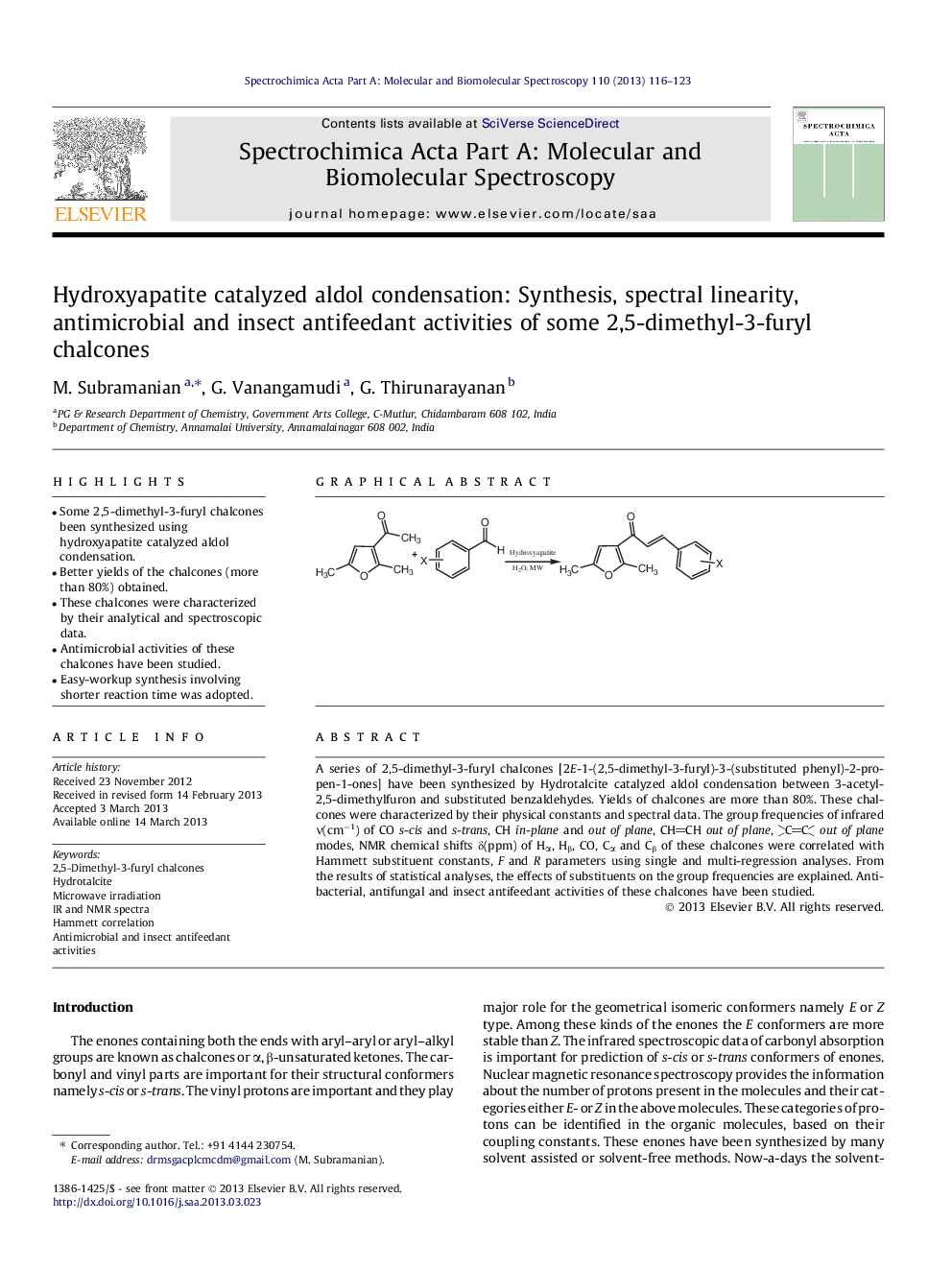
A series of 2,5-dimethyl-3-furyl chalcones [2E-1-(2,5-dimethyl-3-furyl)-3-(substituted phenyl)-2-propen-1-ones] have been synthesized by Hydrotalcite catalyzed aldol condensation between 3-acetyl-2,5-dimethylfuron and substituted benzaldehydes. Yields of chalcones are more than 80%. These chalcones were characterized by their physical constants and spectral data. The group frequencies of infrared ν(cm−1) of CO s-cis and s-trans, CH in-plane and out of plane, CHCH out of plane, CCout of plane modes, NMR chemical shifts δ(ppm) of Hα, Hβ, CO, Cα and Cβ of these chalcones were correlated with Hammett substituent constants, F and R parameters using single and multi-regression analyses. From the results of statistical analyses, the effects of substituents on the group frequencies are explained. Antibacterial, antifungal and insect antifeedant activities of these chalcones have been studied.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide