| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231190 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 7 Pages |
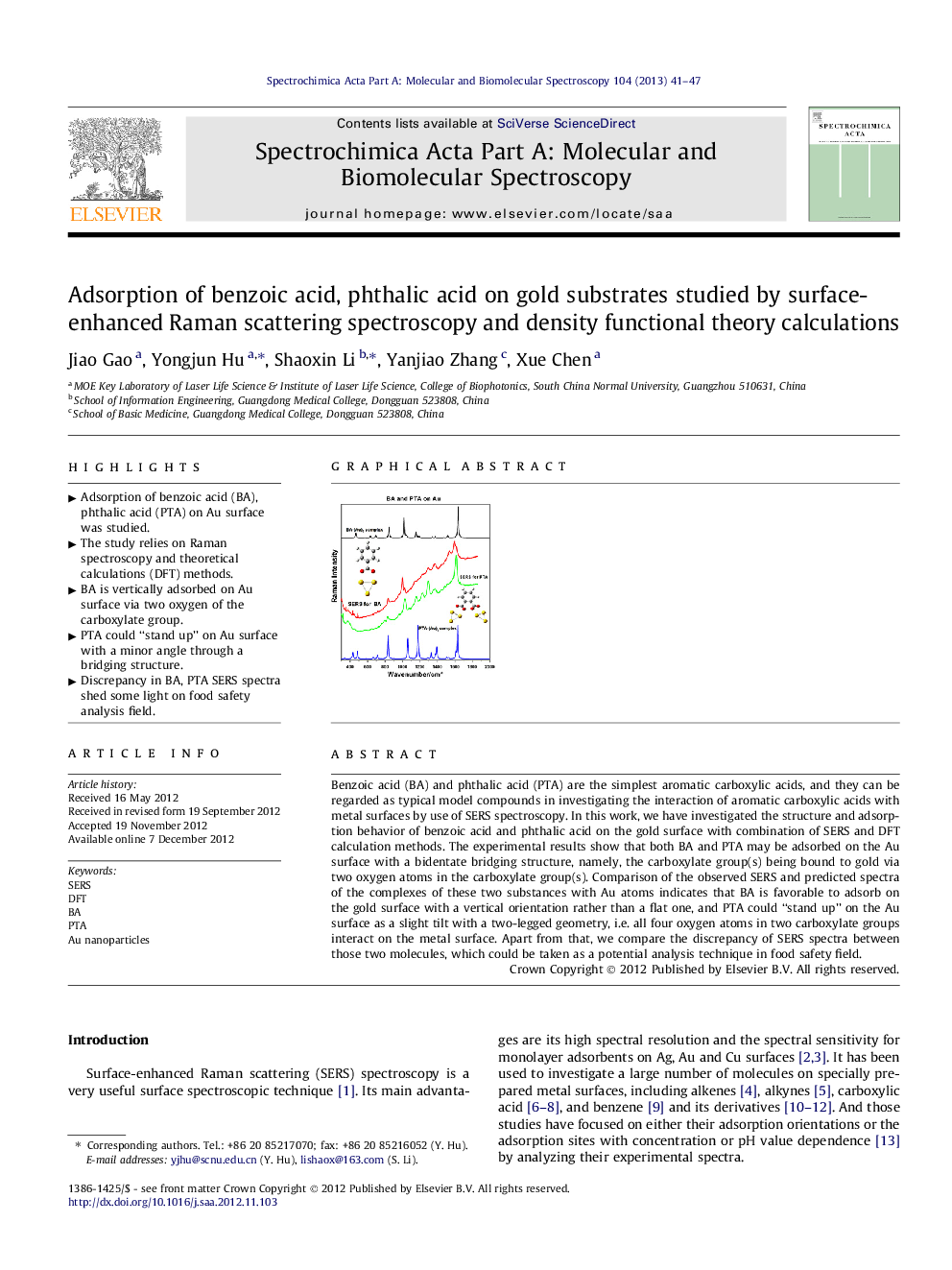
Benzoic acid (BA) and phthalic acid (PTA) are the simplest aromatic carboxylic acids, and they can be regarded as typical model compounds in investigating the interaction of aromatic carboxylic acids with metal surfaces by use of SERS spectroscopy. In this work, we have investigated the structure and adsorption behavior of benzoic acid and phthalic acid on the gold surface with combination of SERS and DFT calculation methods. The experimental results show that both BA and PTA may be adsorbed on the Au surface with a bidentate bridging structure, namely, the carboxylate group(s) being bound to gold via two oxygen atoms in the carboxylate group(s). Comparison of the observed SERS and predicted spectra of the complexes of these two substances with Au atoms indicates that BA is favorable to adsorb on the gold surface with a vertical orientation rather than a flat one, and PTA could “stand up” on the Au surface as a slight tilt with a two-legged geometry, i.e. all four oxygen atoms in two carboxylate groups interact on the metal surface. Apart from that, we compare the discrepancy of SERS spectra between those two molecules, which could be taken as a potential analysis technique in food safety field.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Adsorption of benzoic acid (BA), phthalic acid (PTA) on Au surface was studied. ► The study relies on Raman spectroscopy and theoretical calculations (DFT) methods. ► BA is vertically adsorbed on Au surface via two oxygen of the carboxylate group. ► PTA could “stand up” on Au surface with a minor angle through a bridging structure. ► Discrepancy in BA, PTA SERS spectra shed some light on food safety analysis field.