| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231214 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
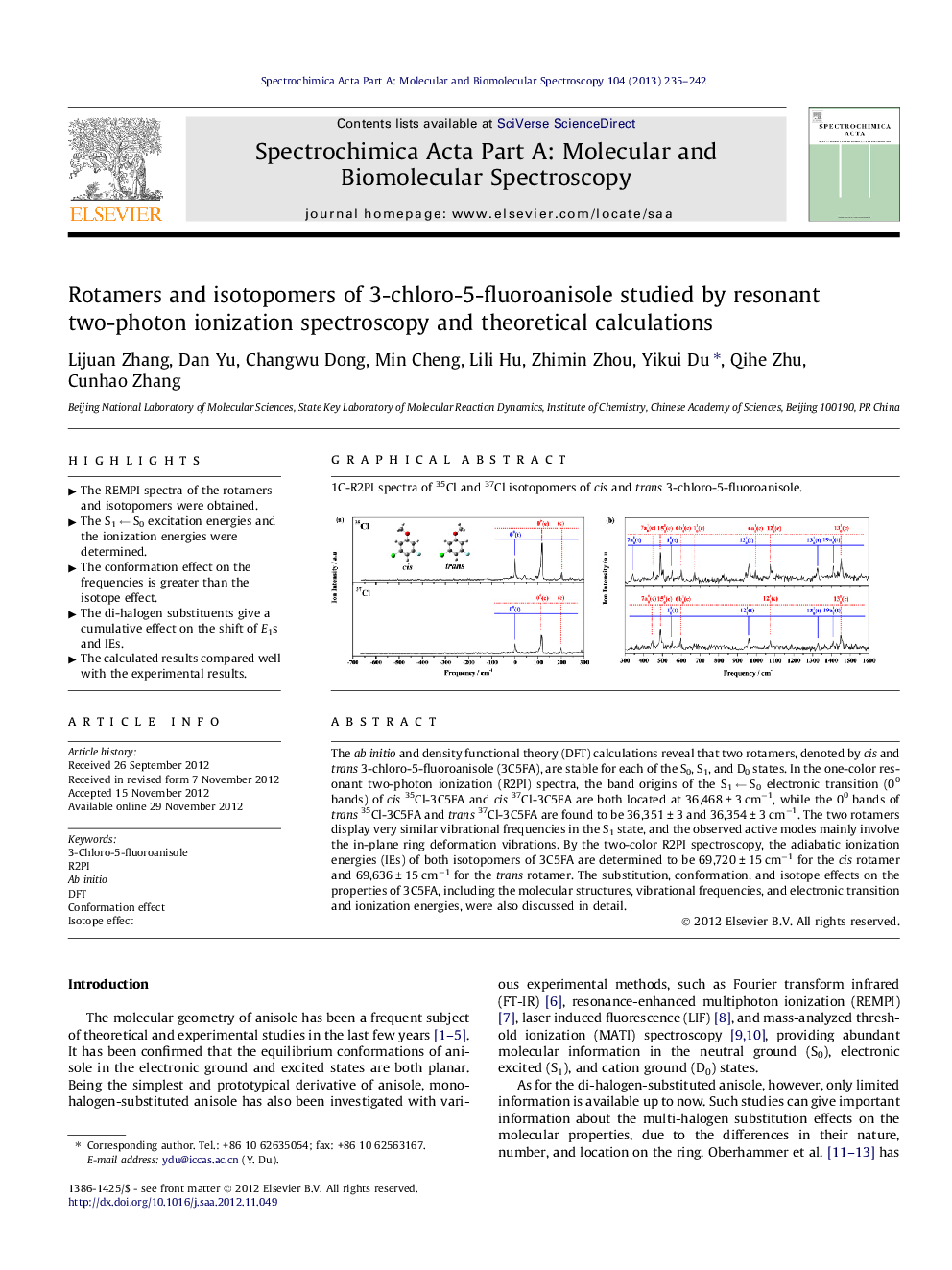
The ab initio and density functional theory (DFT) calculations reveal that two rotamers, denoted by cis and trans 3-chloro-5-fluoroanisole (3C5FA), are stable for each of the S0, S1, and D0 states. In the one-color resonant two-photon ionization (R2PI) spectra, the band origins of the S1 ← S0 electronic transition (00 bands) of cis35Cl-3C5FA and cis37Cl-3C5FA are both located at 36,468 ± 3 cm−1, while the 00 bands of trans35Cl-3C5FA and trans37Cl-3C5FA are found to be 36,351 ± 3 and 36,354 ± 3 cm−1. The two rotamers display very similar vibrational frequencies in the S1 state, and the observed active modes mainly involve the in-plane ring deformation vibrations. By the two-color R2PI spectroscopy, the adiabatic ionization energies (IEs) of both isotopomers of 3C5FA are determined to be 69,720 ± 15 cm−1 for the cis rotamer and 69,636 ± 15 cm−1 for the trans rotamer. The substitution, conformation, and isotope effects on the properties of 3C5FA, including the molecular structures, vibrational frequencies, and electronic transition and ionization energies, were also discussed in detail.
Graphical abstract1C-R2PI spectra of 35Cl and 37Cl isotopomers of cis and trans 3-chloro-5-fluoroanisole.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The REMPI spectra of the rotamers and isotopomers were obtained. ► The S1 ← S0 excitation energies and the ionization energies were determined. ► The conformation effect on the frequencies is greater than the isotope effect. ► The di-halogen substituents give a cumulative effect on the shift of E1s and IEs. ► The calculated results compared well with the experimental results.