| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231268 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
•The electrochemical redox process of molecules was spectrally resolved.•The different interaction modes between molecule and substrate were spectrally distinguished.•Relative Raman intensity and frequency shift are sensitive to interfacial interaction.
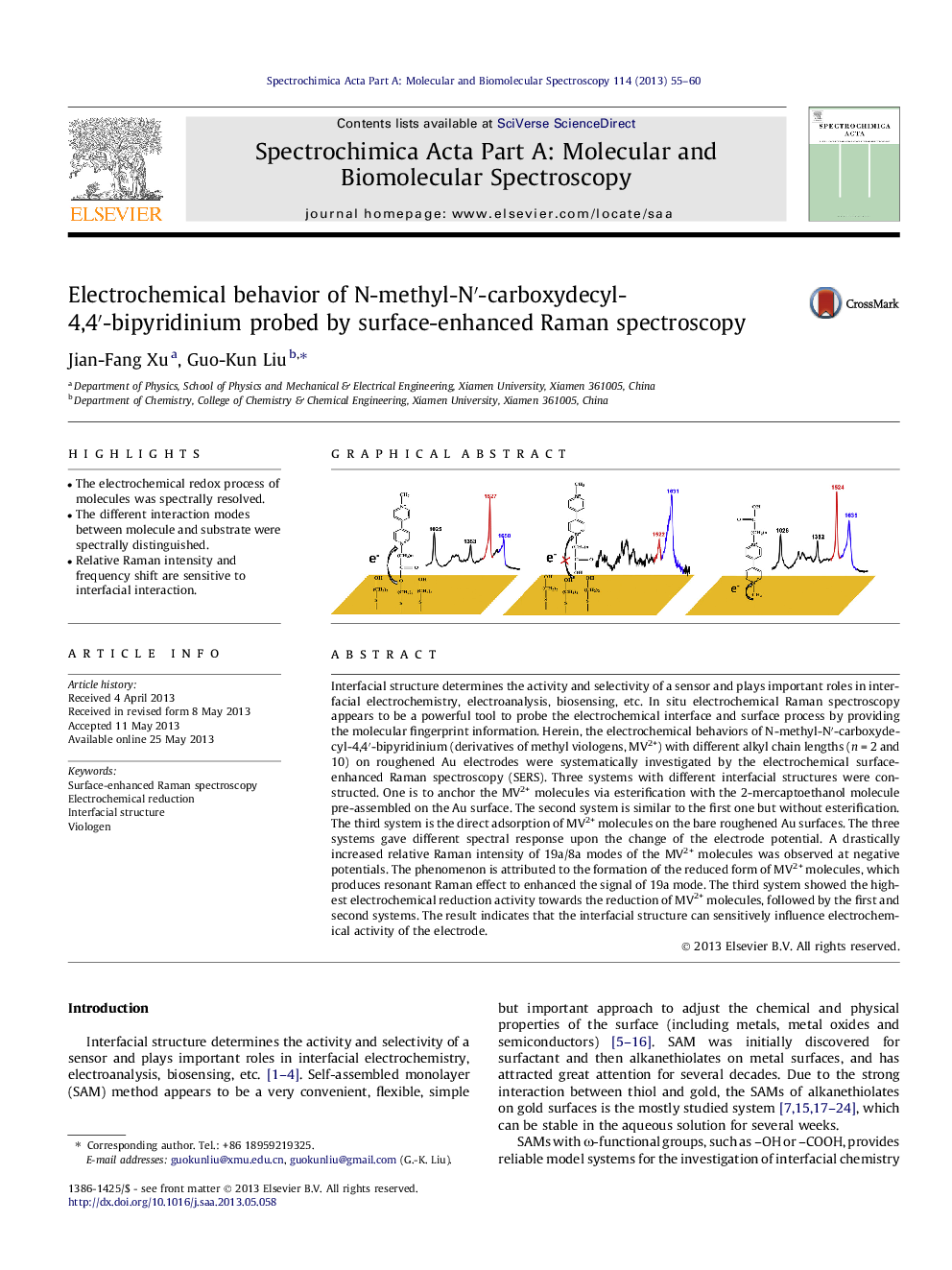
Interfacial structure determines the activity and selectivity of a sensor and plays important roles in interfacial electrochemistry, electroanalysis, biosensing, etc. In situ electrochemical Raman spectroscopy appears to be a powerful tool to probe the electrochemical interface and surface process by providing the molecular fingerprint information. Herein, the electrochemical behaviors of N-methyl-N′-carboxydecyl-4,4′-bipyridinium (derivatives of methyl viologens, MV2+) with different alkyl chain lengths (n = 2 and 10) on roughened Au electrodes were systematically investigated by the electrochemical surface-enhanced Raman spectroscopy (SERS). Three systems with different interfacial structures were constructed. One is to anchor the MV2+ molecules via esterification with the 2-mercaptoethanol molecule pre-assembled on the Au surface. The second system is similar to the first one but without esterification. The third system is the direct adsorption of MV2+ molecules on the bare roughened Au surfaces. The three systems gave different spectral response upon the change of the electrode potential. A drastically increased relative Raman intensity of 19a/8a modes of the MV2+ molecules was observed at negative potentials. The phenomenon is attributed to the formation of the reduced form of MV2+ molecules, which produces resonant Raman effect to enhanced the signal of 19a mode. The third system showed the highest electrochemical reduction activity towards the reduction of MV2+ molecules, followed by the first and second systems. The result indicates that the interfacial structure can sensitively influence electrochemical activity of the electrode.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide