| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231296 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 5 Pages |
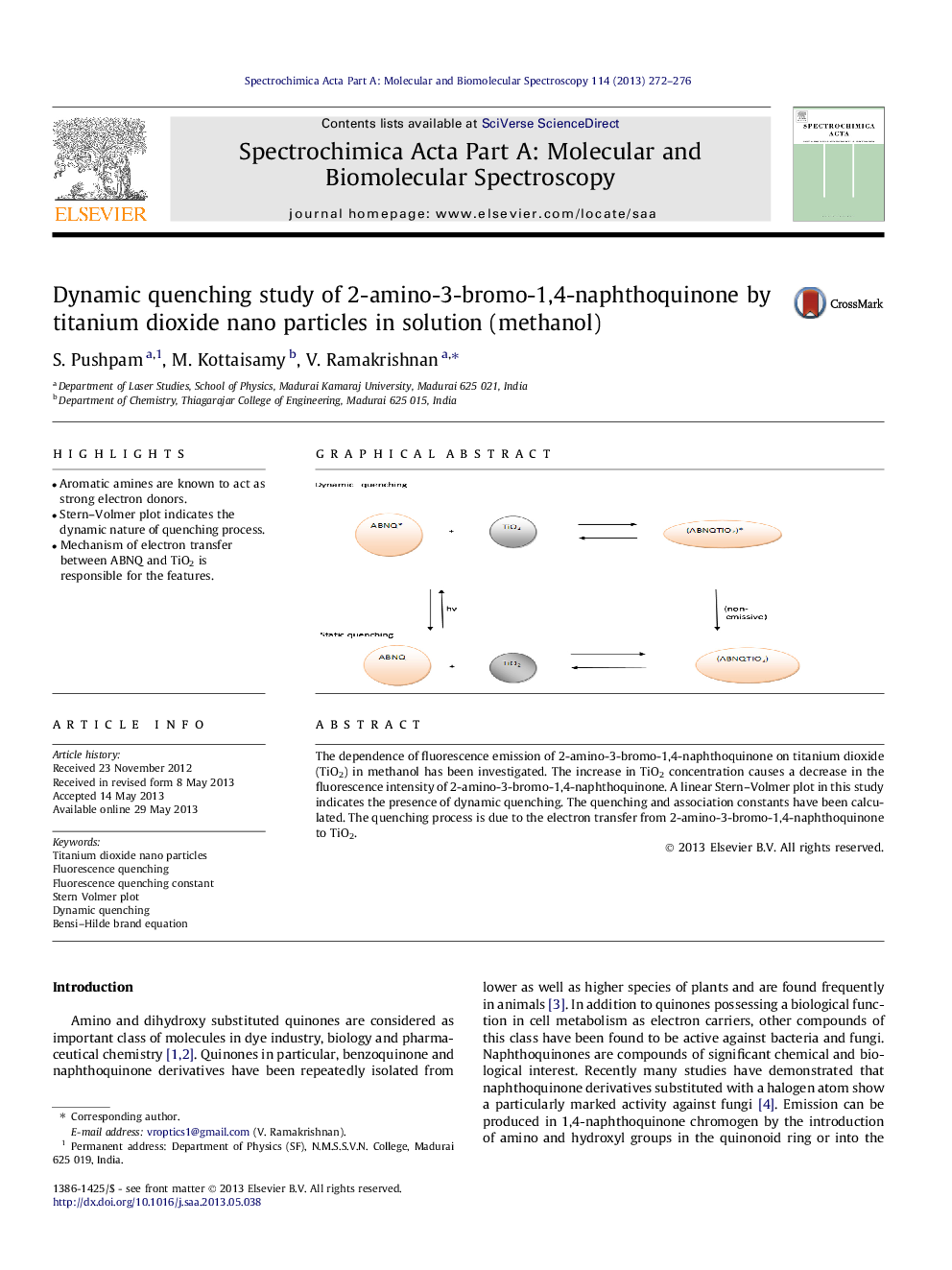
•Aromatic amines are known to act as strong electron donors.•Stern–Volmer plot indicates the dynamic nature of quenching process.•Mechanism of electron transfer between ABNQ and TiO2 is responsible for the features.
The dependence of fluorescence emission of 2-amino-3-bromo-1,4-naphthoquinone on titanium dioxide (TiO2) in methanol has been investigated. The increase in TiO2 concentration causes a decrease in the fluorescence intensity of 2-amino-3-bromo-1,4-naphthoquinone. A linear Stern–Volmer plot in this study indicates the presence of dynamic quenching. The quenching and association constants have been calculated. The quenching process is due to the electron transfer from 2-amino-3-bromo-1,4-naphthoquinone to TiO2.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide