| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231322 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 11 Pages |
•The FT-IR and Raman spectra of AOX are recorded in solid phase.•Theoretical harmonic vibrational frequencies and molecular structure are given for the first time.•The complete assignments are performed on the basis of the total energy distribution (PED).•The HOMO and LUMO energies have been calculated.
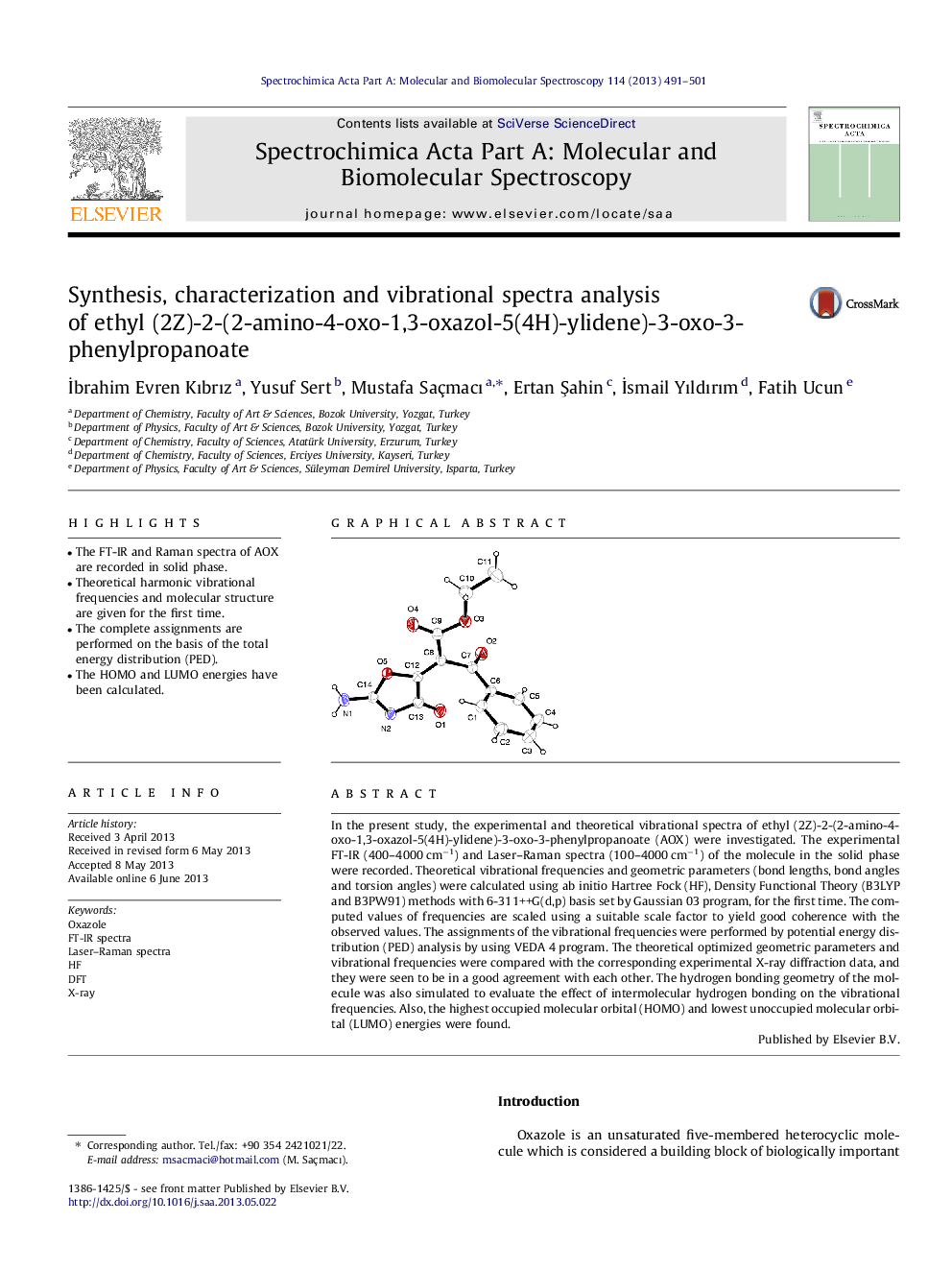
In the present study, the experimental and theoretical vibrational spectra of ethyl (2Z)-2-(2-amino-4-oxo-1,3-oxazol-5(4H)-ylidene)-3-oxo-3-phenylpropanoate (AOX) were investigated. The experimental FT-IR (400–4000 cm−1) and Laser–Raman spectra (100–4000 cm−1) of the molecule in the solid phase were recorded. Theoretical vibrational frequencies and geometric parameters (bond lengths, bond angles and torsion angles) were calculated using ab initio Hartree Fock (HF), Density Functional Theory (B3LYP and B3PW91) methods with 6-311++G(d,p) basis set by Gaussian 03 program, for the first time. The computed values of frequencies are scaled using a suitable scale factor to yield good coherence with the observed values. The assignments of the vibrational frequencies were performed by potential energy distribution (PED) analysis by using VEDA 4 program. The theoretical optimized geometric parameters and vibrational frequencies were compared with the corresponding experimental X-ray diffraction data, and they were seen to be in a good agreement with each other. The hydrogen bonding geometry of the molecule was also simulated to evaluate the effect of intermolecular hydrogen bonding on the vibrational frequencies. Also, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies were found.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide