| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231325 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 11 Pages |
•Study of molecular interactions between hydrogen bonded polar liquid systems.•Liquids of propan-1-ol, propionaldehyde and its binary mixtures are considered.•Dielectric relaxation studies in the microwave frequency region at different temp.•Conformational analysis by FT-IR spectra and ab-initio calculations.•Evaluation of dielectric and thermodynamic parameters.
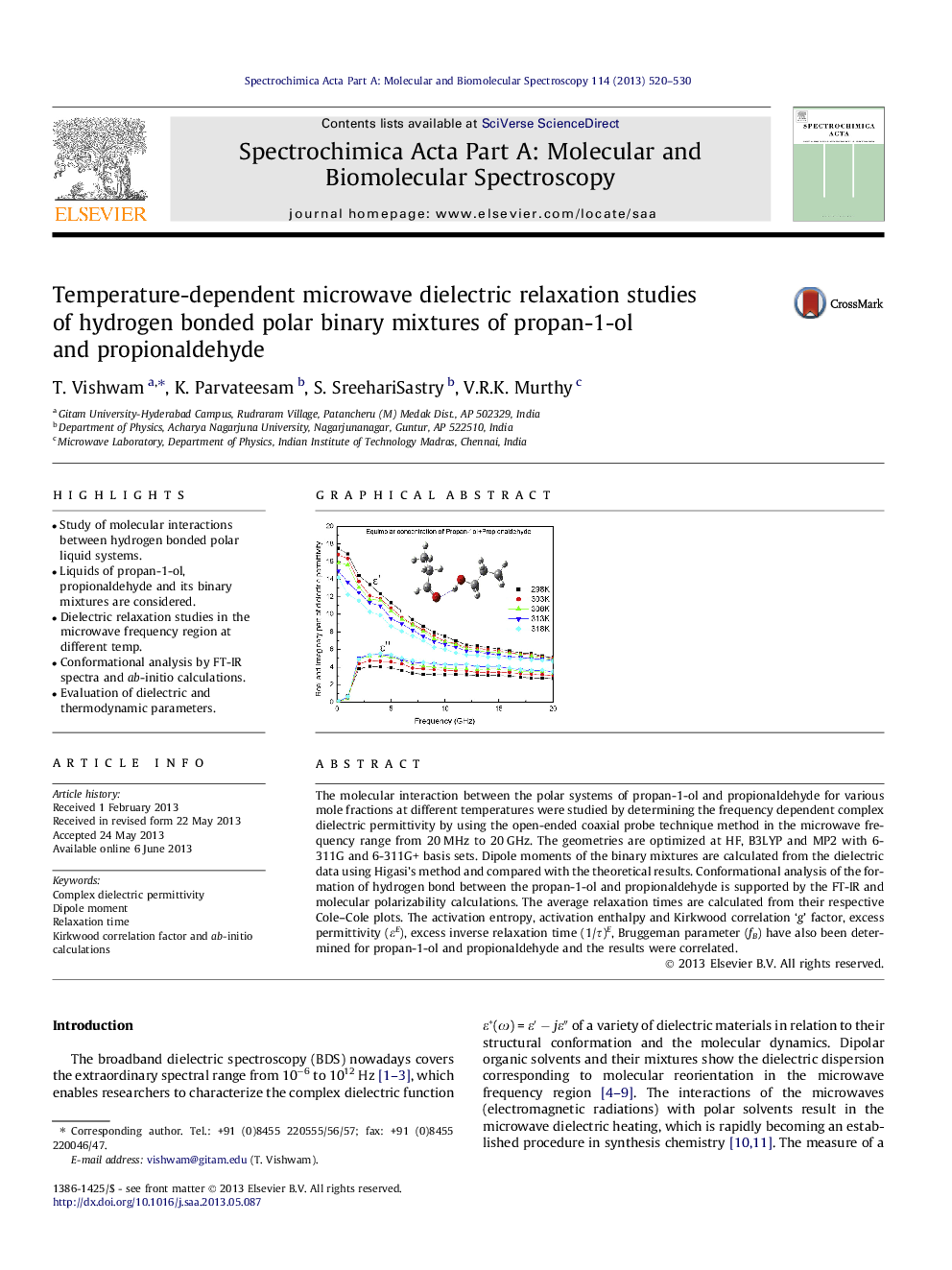
The molecular interaction between the polar systems of propan-1-ol and propionaldehyde for various mole fractions at different temperatures were studied by determining the frequency dependent complex dielectric permittivity by using the open-ended coaxial probe technique method in the microwave frequency range from 20 MHz to 20 GHz. The geometries are optimized at HF, B3LYP and MP2 with 6-311G and 6-311G+ basis sets. Dipole moments of the binary mixtures are calculated from the dielectric data using Higasi’s method and compared with the theoretical results. Conformational analysis of the formation of hydrogen bond between the propan-1-ol and propionaldehyde is supported by the FT-IR and molecular polarizability calculations. The average relaxation times are calculated from their respective Cole–Cole plots. The activation entropy, activation enthalpy and Kirkwood correlation ‘g’ factor, excess permittivity (εE), excess inverse relaxation time (1/τ)E, Bruggeman parameter (fB) have also been determined for propan-1-ol and propionaldehyde and the results were correlated.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide