| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231459 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 7 Pages |
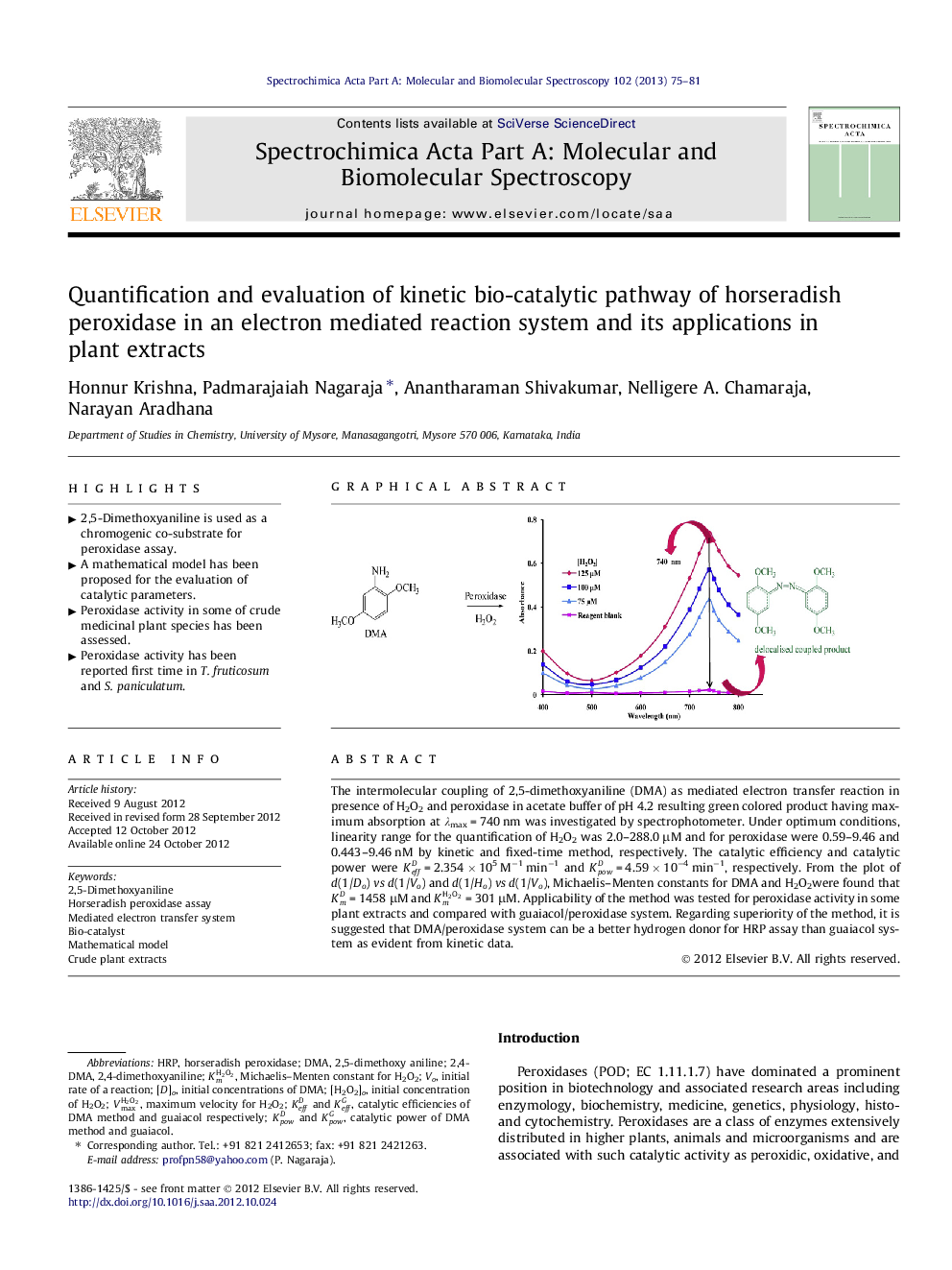
The intermolecular coupling of 2,5-dimethoxyaniline (DMA) as mediated electron transfer reaction in presence of H2O2 and peroxidase in acetate buffer of pH 4.2 resulting green colored product having maximum absorption at λmax = 740 nm was investigated by spectrophotometer. Under optimum conditions, linearity range for the quantification of H2O2 was 2.0–288.0 μM and for peroxidase were 0.59–9.46 and 0.443–9.46 nM by kinetic and fixed-time method, respectively. The catalytic efficiency and catalytic power were KeffD = 2.354 × 105 M−1 min−1 and KpowD = 4.59 × 10−4 min−1, respectively. From the plot of d(1/Do) vs d(1/Vo) and d(1/Ho) vs d(1/Vo), Michaelis–Menten constants for DMA and H2O2were found that KmD = 1458 μM and KmH2O2 = 301 μM. Applicability of the method was tested for peroxidase activity in some plant extracts and compared with guaiacol/peroxidase system. Regarding superiority of the method, it is suggested that DMA/peroxidase system can be a better hydrogen donor for HRP assay than guaiacol system as evident from kinetic data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► 2,5-Dimethoxyaniline is used as a chromogenic co-substrate for peroxidase assay. ► A mathematical model has been proposed for the evaluation of catalytic parameters. ► Peroxidase activity in some of crude medicinal plant species has been assessed. ► Peroxidase activity has been reported first time in T. fruticosum and S. paniculatum.