| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231471 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 11 Pages |
Copper guanine and barbital complexes were prepared and characterized by elemental analyses and spectral measurements. The data typified the formation of stoichiometries 1:1 (M:L) with possible Cu–Cu interaction “association”. The complexes are with different geometries: square planar, square pyramidal and tetrahedral. The mode of bonding was identified by IR spectra. EPR spectra of the powdered complexes were recorded at X band at the room temperature. Different ESR parameters were calculated and discussed: g//, g⊥, A//, 〈g〉, G, F, K, α2. Molecular modeling techniques and quantum chemical methods have been performed for copper complexes to correlate the chemical structures of the complexes with their physical molecular properties. Bond lengths, bond orders, bond angles, dihedral angles, close contact, dipole moment (μ), sum of the total negative charge (STNC), electronegativity (χ), chemical potential (Pi), global hardness (η), softness (σ), the highest occupied molecular orbital energy (EHOMO), the lowest unoccupied molecular orbital energy (ELUMO) and the energy gap (ΔE) were calculated using PM3 semi-empirical and Molecular Mechanics (MM+) methods. The study displays a good correlation between the theoretical and experimental data which confirms the reliability of the quantum chemical methods.
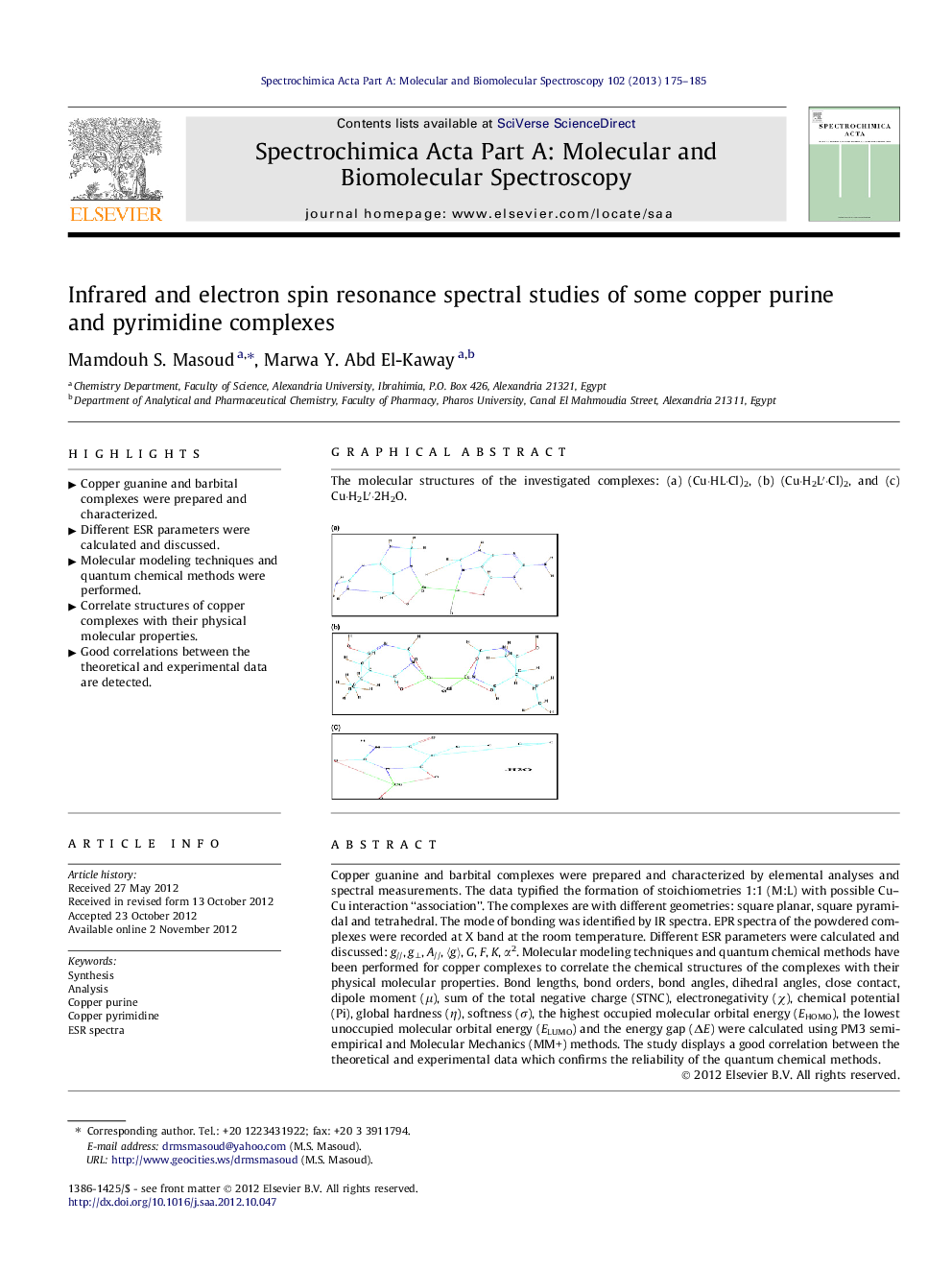
Graphical abstractThe molecular structures of the investigated complexes: (a) (Cu·HL·Cl)2, (b) (Cu·H2L′·Cl)2, and (c) Cu·H2L′·2H2O.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Copper guanine and barbital complexes were prepared and characterized. ► Different ESR parameters were calculated and discussed. ► Molecular modeling techniques and quantum chemical methods were performed. ► Correlate structures of copper complexes with their physical molecular properties. ► Good correlations between the theoretical and experimental data are detected.