| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231472 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
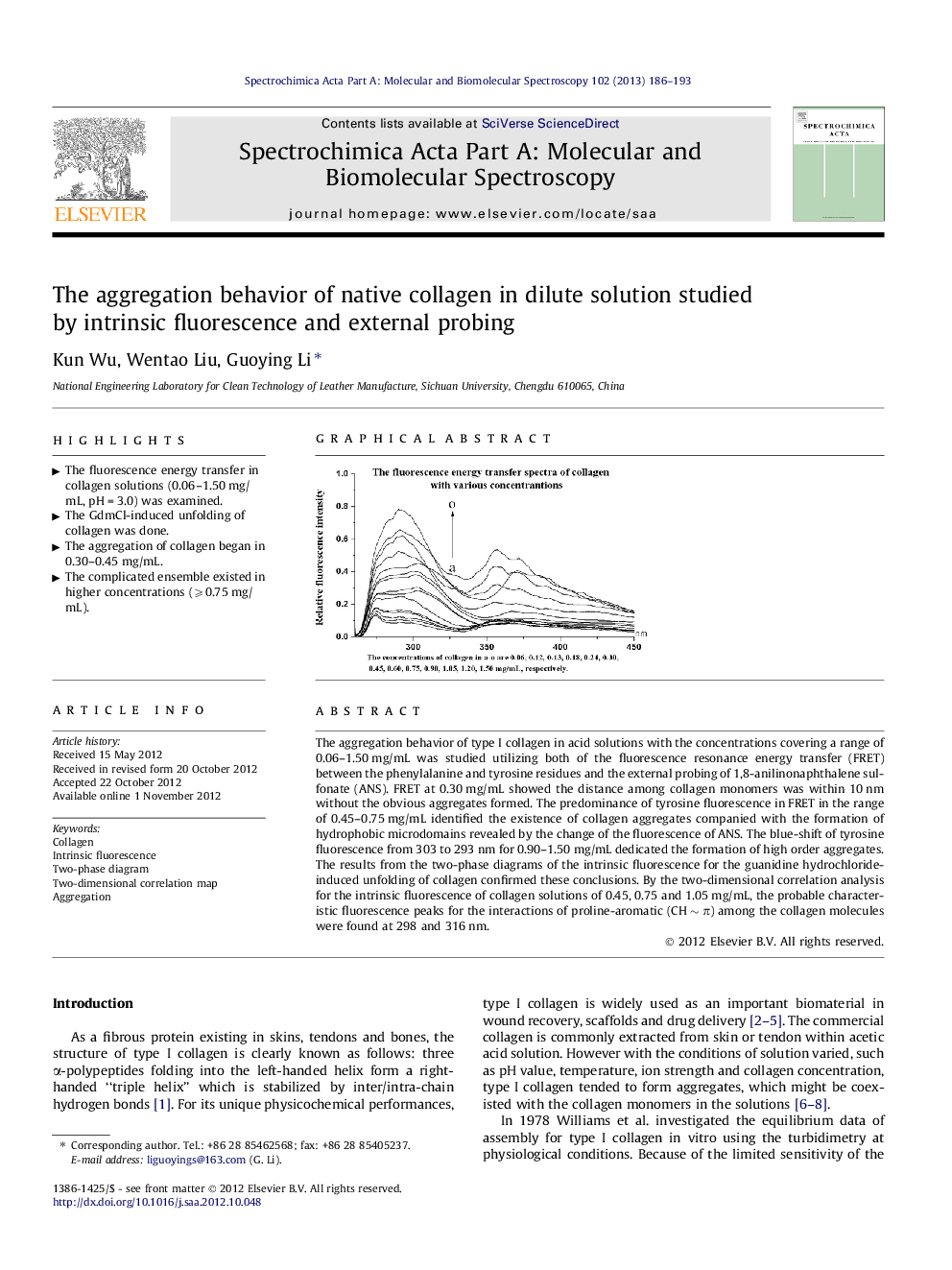
The aggregation behavior of type I collagen in acid solutions with the concentrations covering a range of 0.06–1.50 mg/mL was studied utilizing both of the fluorescence resonance energy transfer (FRET) between the phenylalanine and tyrosine residues and the external probing of 1,8-anilinonaphthalene sulfonate (ANS). FRET at 0.30 mg/mL showed the distance among collagen monomers was within 10 nm without the obvious aggregates formed. The predominance of tyrosine fluorescence in FRET in the range of 0.45–0.75 mg/mL identified the existence of collagen aggregates companied with the formation of hydrophobic microdomains revealed by the change of the fluorescence of ANS. The blue-shift of tyrosine fluorescence from 303 to 293 nm for 0.90–1.50 mg/mL dedicated the formation of high order aggregates. The results from the two-phase diagrams of the intrinsic fluorescence for the guanidine hydrochloride-induced unfolding of collagen confirmed these conclusions. By the two-dimensional correlation analysis for the intrinsic fluorescence of collagen solutions of 0.45, 0.75 and 1.05 mg/mL, the probable characteristic fluorescence peaks for the interactions of proline-aromatic (CH ∼ π) among the collagen molecules were found at 298 and 316 nm.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The fluorescence energy transfer in collagen solutions (0.06–1.50 mg/mL, pH = 3.0) was examined. ► The GdmCl-induced unfolding of collagen was done. ► The aggregation of collagen began in 0.30–0.45 mg/mL. ► The complicated ensemble existed in higher concentrations (⩾0.75 mg/mL).