| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231501 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 5 Pages |
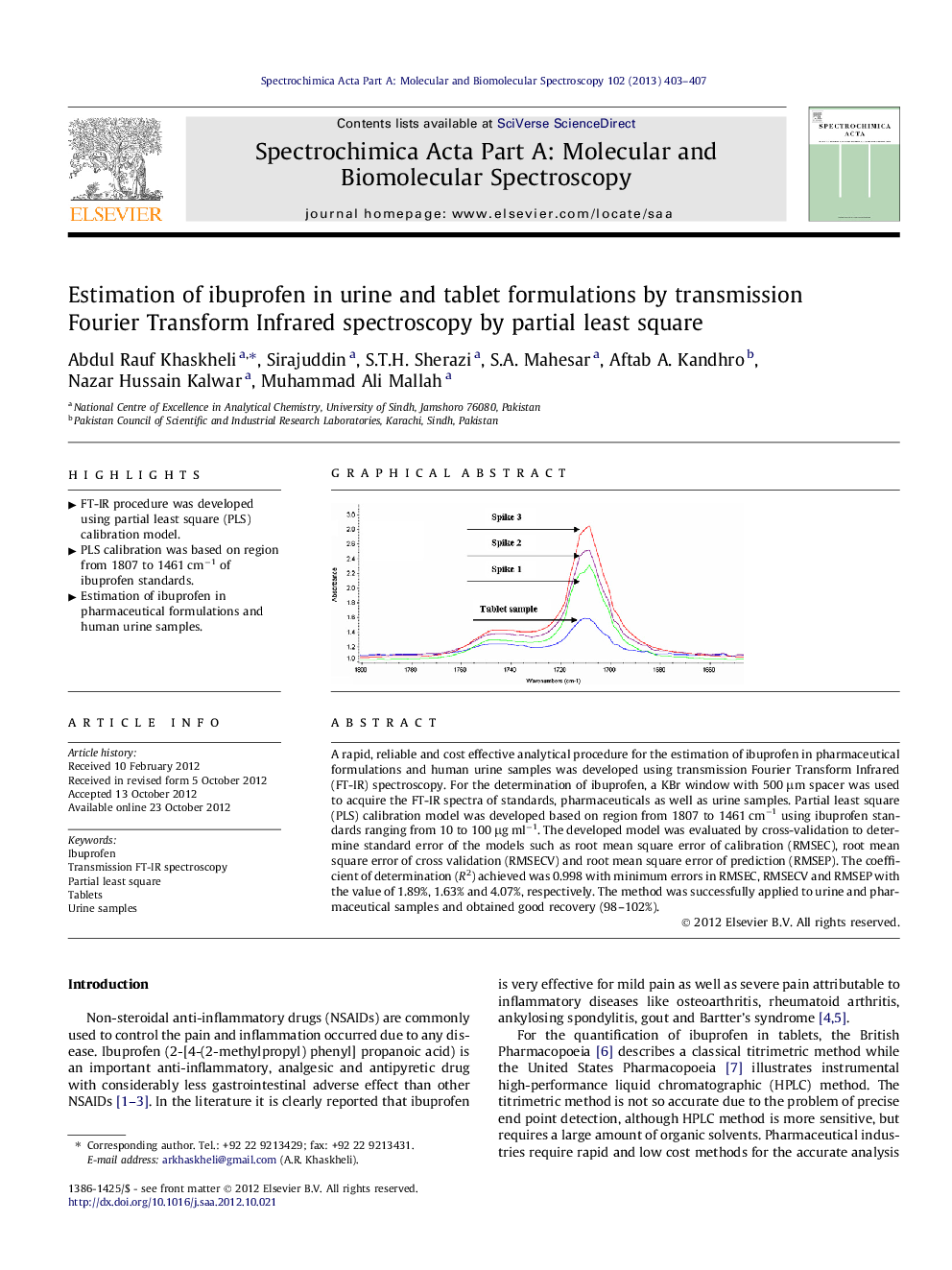
A rapid, reliable and cost effective analytical procedure for the estimation of ibuprofen in pharmaceutical formulations and human urine samples was developed using transmission Fourier Transform Infrared (FT-IR) spectroscopy. For the determination of ibuprofen, a KBr window with 500 μm spacer was used to acquire the FT-IR spectra of standards, pharmaceuticals as well as urine samples. Partial least square (PLS) calibration model was developed based on region from 1807 to 1461 cm−1 using ibuprofen standards ranging from 10 to 100 μg ml−1. The developed model was evaluated by cross-validation to determine standard error of the models such as root mean square error of calibration (RMSEC), root mean square error of cross validation (RMSECV) and root mean square error of prediction (RMSEP). The coefficient of determination (R2) achieved was 0.998 with minimum errors in RMSEC, RMSECV and RMSEP with the value of 1.89%, 1.63% and 4.07%, respectively. The method was successfully applied to urine and pharmaceutical samples and obtained good recovery (98–102%).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► FT-IR procedure was developed using partial least square (PLS) calibration model. ► PLS calibration was based on region from 1807 to 1461 cm−1 of ibuprofen standards. ► Estimation of ibuprofen in pharmaceutical formulations and human urine samples.