| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231504 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
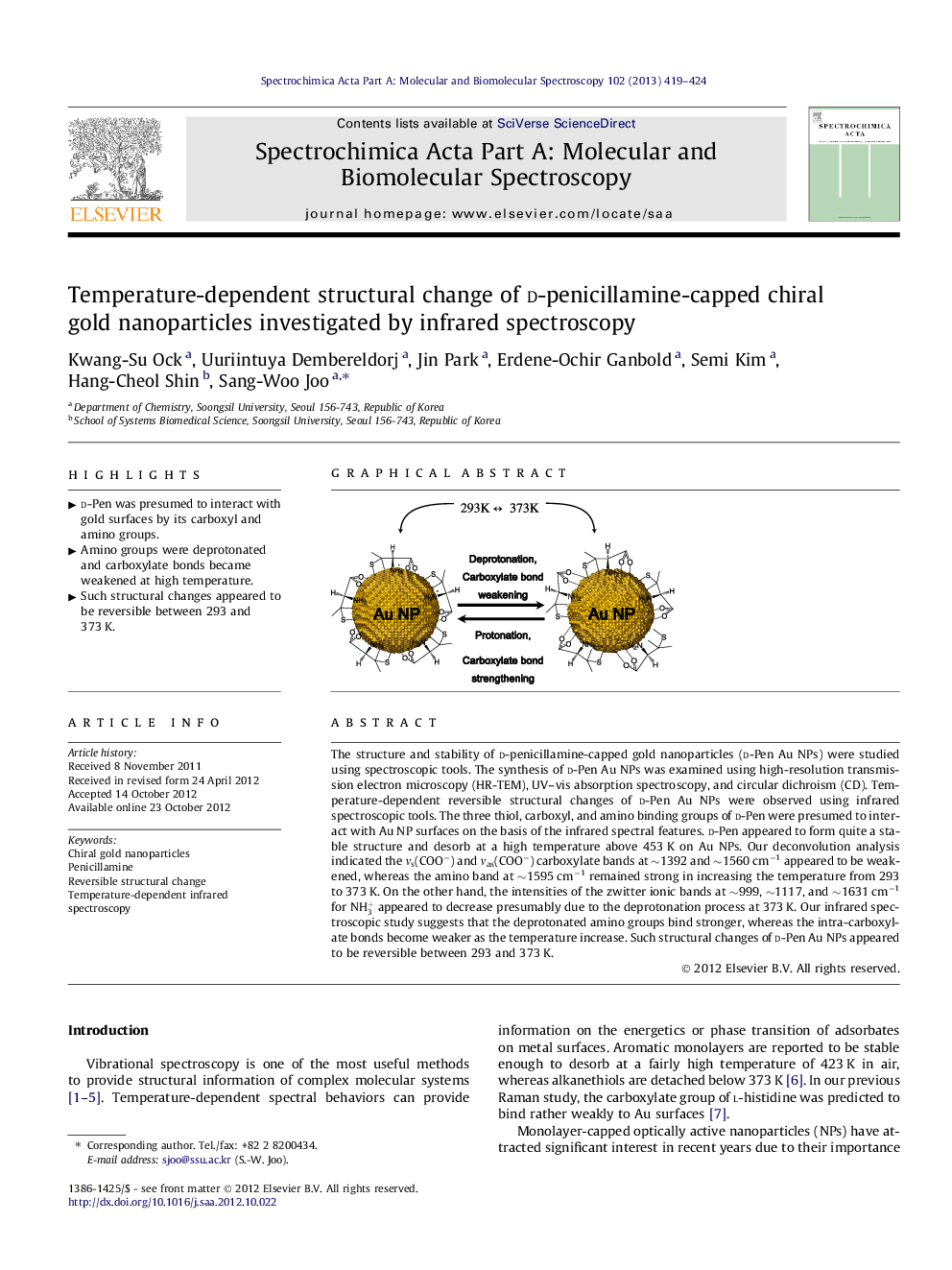
The structure and stability of d-penicillamine-capped gold nanoparticles (d-Pen Au NPs) were studied using spectroscopic tools. The synthesis of d-Pen Au NPs was examined using high-resolution transmission electron microscopy (HR-TEM), UV–vis absorption spectroscopy, and circular dichroism (CD). Temperature-dependent reversible structural changes of d-Pen Au NPs were observed using infrared spectroscopic tools. The three thiol, carboxyl, and amino binding groups of d-Pen were presumed to interact with Au NP surfaces on the basis of the infrared spectral features. d-Pen appeared to form quite a stable structure and desorb at a high temperature above 453 K on Au NPs. Our deconvolution analysis indicated the νs(COO−) and νas(COO−) carboxylate bands at ∼1392 and ∼1560 cm−1 appeared to be weakened, whereas the amino band at ∼1595 cm−1 remained strong in increasing the temperature from 293 to 373 K. On the other hand, the intensities of the zwitter ionic bands at ∼999, ∼1117, and ∼1631 cm−1 for NH3+ appeared to decrease presumably due to the deprotonation process at 373 K. Our infrared spectroscopic study suggests that the deprotonated amino groups bind stronger, whereas the intra-carboxylate bonds become weaker as the temperature increase. Such structural changes of d-Pen Au NPs appeared to be reversible between 293 and 373 K.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► d-Pen was presumed to interact with gold surfaces by its carboxyl and amino groups. ► Amino groups were deprotonated and carboxylate bonds became weakened at high temperature. ► Such structural changes appeared to be reversible between 293 and 373 K.