| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231601 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
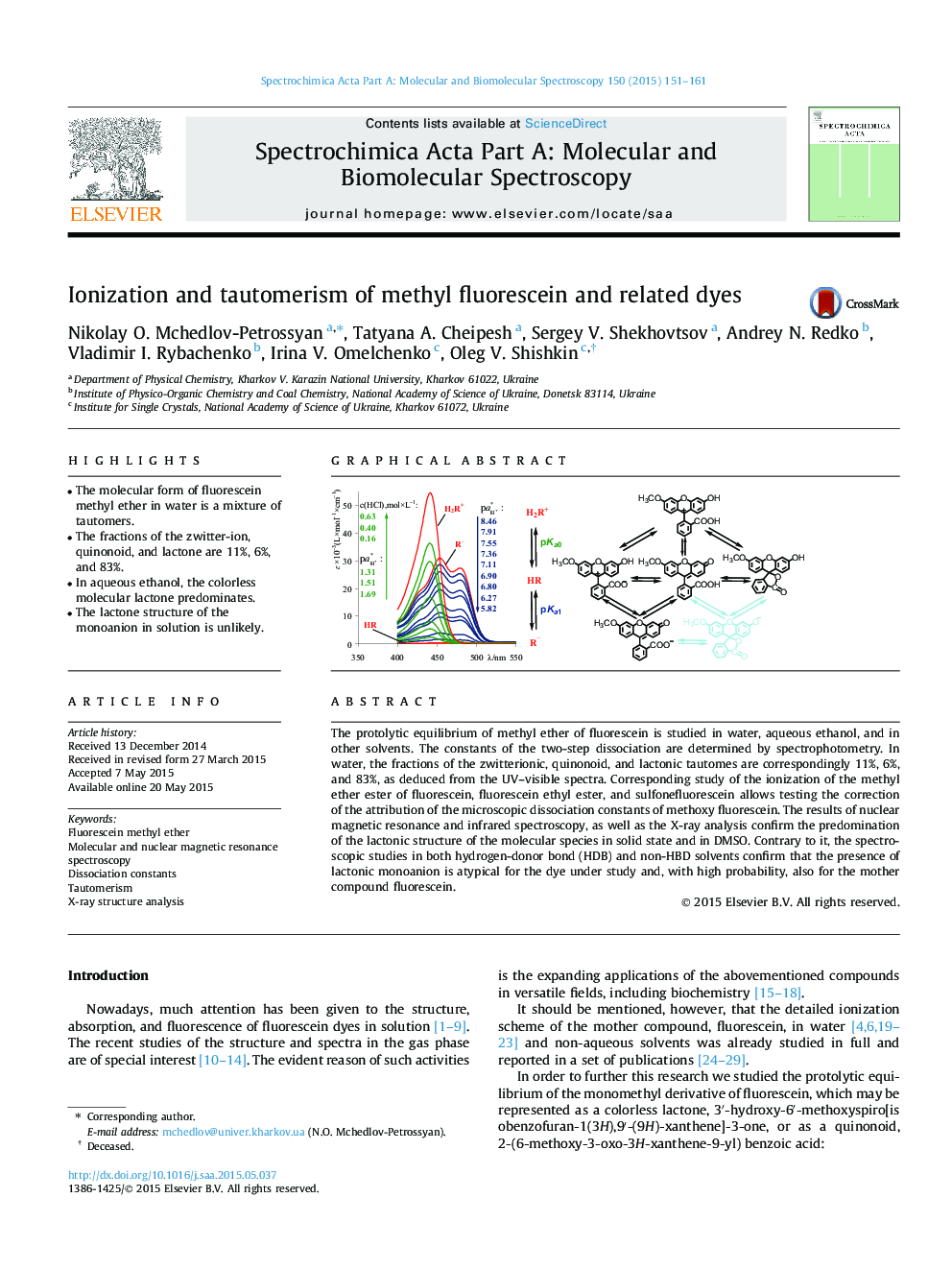
•The molecular form of fluorescein methyl ether in water is a mixture of tautomers.•The fractions of the zwitter-ion, quinonoid, and lactone are 11%, 6%, and 83%.•In aqueous ethanol, the colorless molecular lactone predominates.•The lactone structure of the monoanion in solution is unlikely.
The protolytic equilibrium of methyl ether of fluorescein is studied in water, aqueous ethanol, and in other solvents. The constants of the two-step dissociation are determined by spectrophotometry. In water, the fractions of the zwitterionic, quinonoid, and lactonic tautomes are correspondingly 11%, 6%, and 83%, as deduced from the UV–visible spectra. Corresponding study of the ionization of the methyl ether ester of fluorescein, fluorescein ethyl ester, and sulfonefluorescein allows testing the correction of the attribution of the microscopic dissociation constants of methoxy fluorescein. The results of nuclear magnetic resonance and infrared spectroscopy, as well as the X-ray analysis confirm the predomination of the lactonic structure of the molecular species in solid state and in DMSO. Contrary to it, the spectroscopic studies in both hydrogen-donor bond (HDB) and non-HBD solvents confirm that the presence of lactonic monoanion is atypical for the dye under study and, with high probability, also for the mother compound fluorescein.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide