| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231615 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
•A novel cyclobutane compound was synthesized.•It was brought to light the molecular structure by X-ray diffraction.•The compound was characterized by FT-IR and NMR spectroscopies.•Geometric parameters, vibrational frequencies and chemical shifts were calculated by DFT methods.•Experimental data was supported by theoretical and literature values.
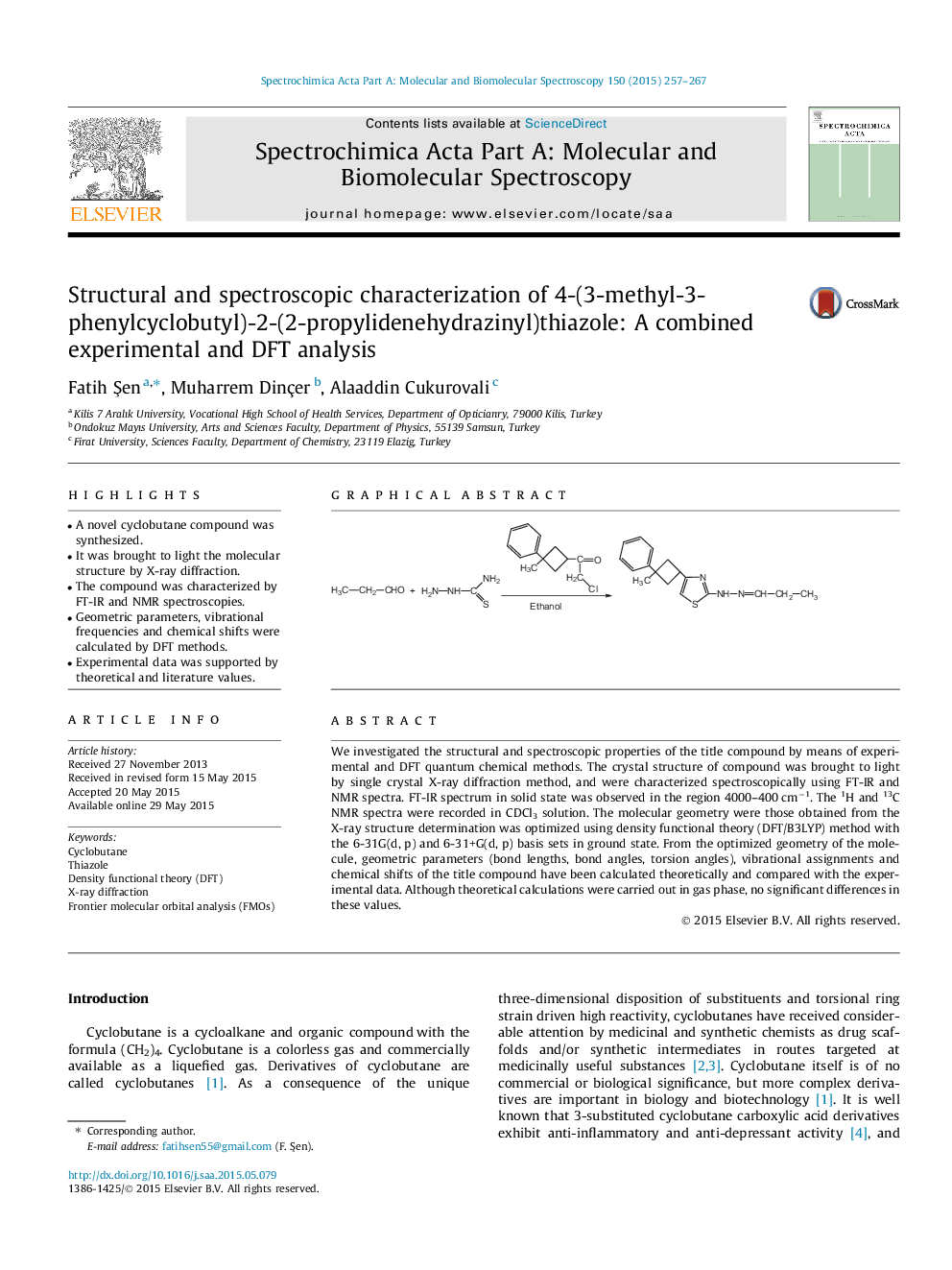
We investigated the structural and spectroscopic properties of the title compound by means of experimental and DFT quantum chemical methods. The crystal structure of compound was brought to light by single crystal X-ray diffraction method, and were characterized spectroscopically using FT-IR and NMR spectra. FT-IR spectrum in solid state was observed in the region 4000–400 cm−1. The 1H and 13C NMR spectra were recorded in CDCl3 solution. The molecular geometry were those obtained from the X-ray structure determination was optimized using density functional theory (DFT/B3LYP) method with the 6-31G(d, p) and 6-31+G(d, p) basis sets in ground state. From the optimized geometry of the molecule, geometric parameters (bond lengths, bond angles, torsion angles), vibrational assignments and chemical shifts of the title compound have been calculated theoretically and compared with the experimental data. Although theoretical calculations were carried out in gas phase, no significant differences in these values.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide