| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231618 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
•Four new Cu(II) Schiff-base complexes 1–4 were synthesized and characterized.•The interactions of 1–4 with HSA have been investigated.•1–4 quench the fluorescence of HSA through a static quenching mechanism.•The active binding sites for 1, 2 and 4 are site III in IB and for 3 is site II in III A.•1–4 bind to HSA through hydrophobic forces as well as hydrogen bonds.
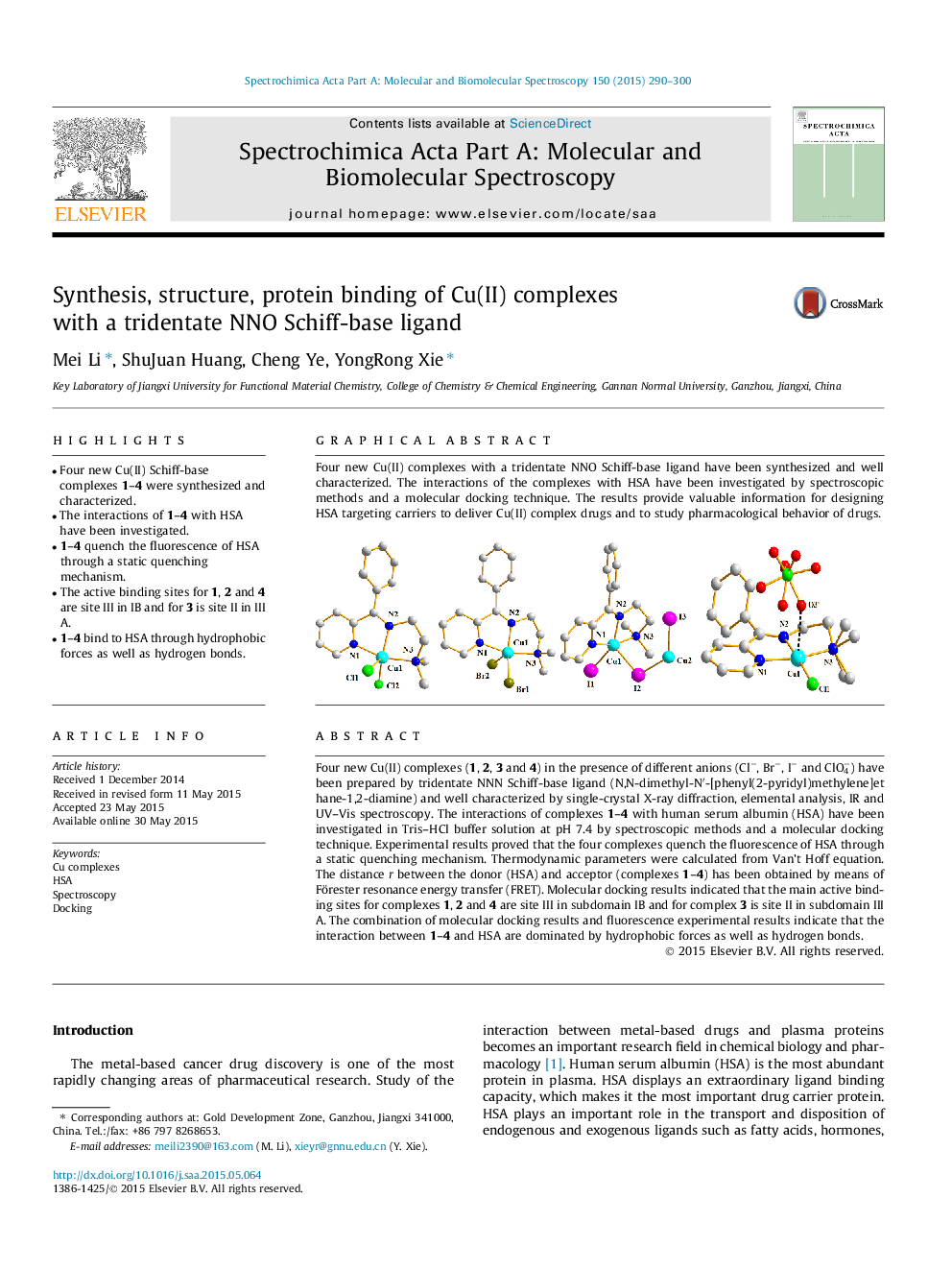
Four new Cu(II) complexes (1, 2, 3 and 4) in the presence of different anions (Cl−, Br−, I− and ClO4−) have been prepared by tridentate NNN Schiff-base ligand (N,N-dimethyl-N′-[phenyl(2-pyridyl)methylene]ethane-1,2-diamine) and well characterized by single-crystal X-ray diffraction, elemental analysis, IR and UV–Vis spectroscopy. The interactions of complexes 1–4 with human serum albumin (HSA) have been investigated in Tris–HCl buffer solution at pH 7.4 by spectroscopic methods and a molecular docking technique. Experimental results proved that the four complexes quench the fluorescence of HSA through a static quenching mechanism. Thermodynamic parameters were calculated from Van’t Hoff equation. The distance r between the donor (HSA) and acceptor (complexes 1–4) has been obtained by means of Förester resonance energy transfer (FRET). Molecular docking results indicated that the main active binding sites for complexes 1, 2 and 4 are site III in subdomain IB and for complex 3 is site II in subdomain III A. The combination of molecular docking results and fluorescence experimental results indicate that the interaction between 1–4 and HSA are dominated by hydrophobic forces as well as hydrogen bonds.
Graphical abstractFour new Cu(II) complexes with a tridentate NNO Schiff-base ligand have been synthesized and well characterized. The interactions of the complexes with HSA have been investigated by spectroscopic methods and a molecular docking technique. The results provide valuable information for designing HSA targeting carriers to deliver Cu(II) complex drugs and to study pharmacological behavior of drugs.Figure optionsDownload full-size imageDownload as PowerPoint slide