| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231623 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
•The plasmon induced conversion of PNTP was dependent on the laser power, solvent and substrate structure.•Water played the essential role in the plasmon catalyzed reaction of PNTP in ionic liquids.•The introduction of coadsorption species resulted in the inhibition on dimerization of PNTP.•The activity of plasmon catalyzed dimerization of PNTP was in the sequence of H2O > methanol > n-hexane ≈ acetonitrile ≈ DMF.
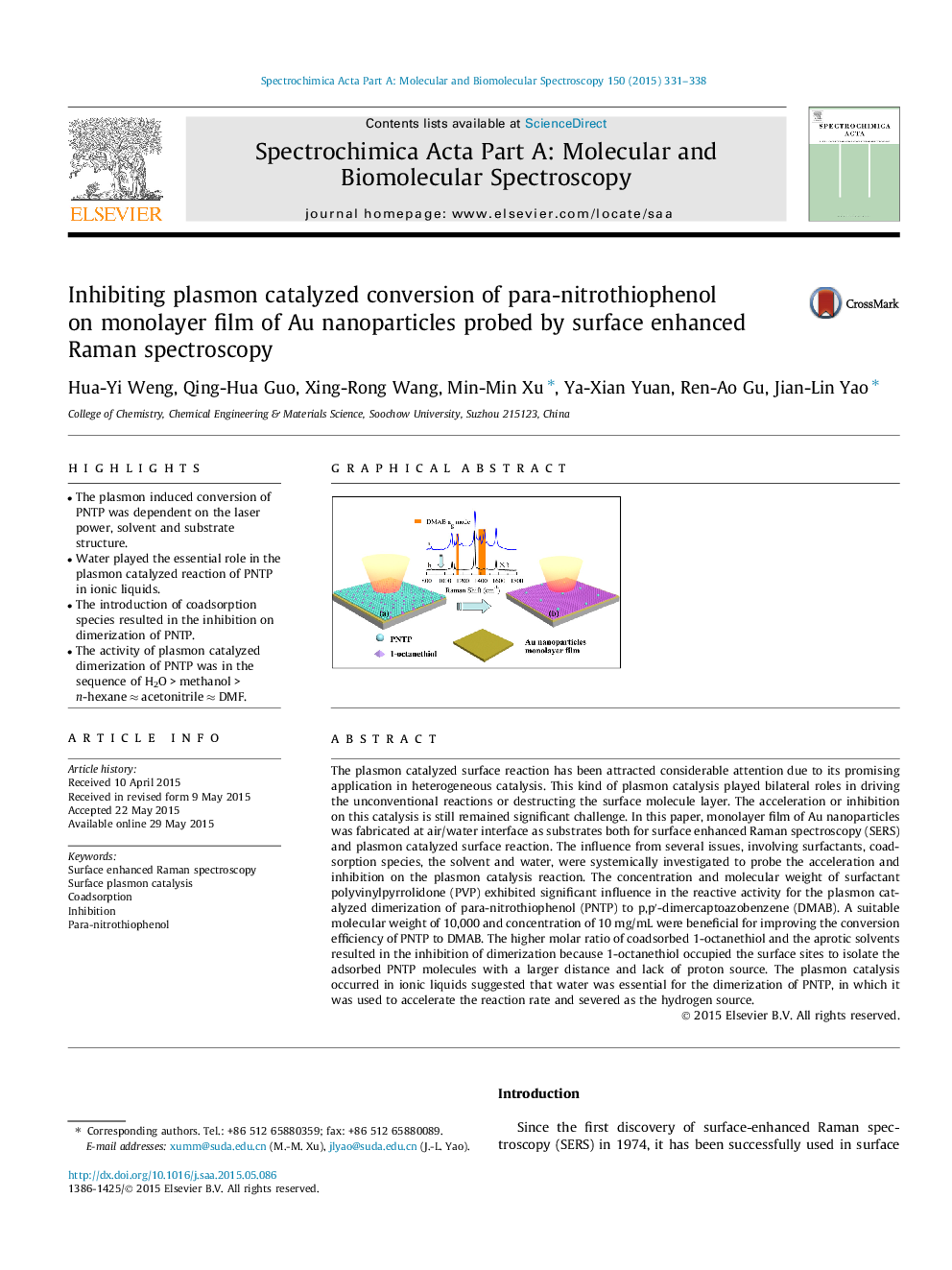
The plasmon catalyzed surface reaction has been attracted considerable attention due to its promising application in heterogeneous catalysis. This kind of plasmon catalysis played bilateral roles in driving the unconventional reactions or destructing the surface molecule layer. The acceleration or inhibition on this catalysis is still remained significant challenge. In this paper, monolayer film of Au nanoparticles was fabricated at air/water interface as substrates both for surface enhanced Raman spectroscopy (SERS) and plasmon catalyzed surface reaction. The influence from several issues, involving surfactants, coadsorption species, the solvent and water, were systemically investigated to probe the acceleration and inhibition on the plasmon catalysis reaction. The concentration and molecular weight of surfactant polyvinylpyrrolidone (PVP) exhibited significant influence in the reactive activity for the plasmon catalyzed dimerization of para-nitrothiophenol (PNTP) to p,p′-dimercaptoazobenzene (DMAB). A suitable molecular weight of 10,000 and concentration of 10 mg/mL were beneficial for improving the conversion efficiency of PNTP to DMAB. The higher molar ratio of coadsorbed 1-octanethiol and the aprotic solvents resulted in the inhibition of dimerization because 1-octanethiol occupied the surface sites to isolate the adsorbed PNTP molecules with a larger distance and lack of proton source. The plasmon catalysis occurred in ionic liquids suggested that water was essential for the dimerization of PNTP, in which it was used to accelerate the reaction rate and severed as the hydrogen source.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide