| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231852 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
Some tetradentate salen type Schiff bases and their uranyl complexes were synthesized and characterized by UV–Vis, NMR, IR, TG, C.H.N. and X-ray crystallographic studies. From these investigations it is confirmed that a solvent molecule occupied the fifth position of the equatorial plane of the distorted pentagonal bipyramidal structure. Also, the kinetics of complex decomposition by using thermo gravimetric methods (TG) was studied. The thermal decomposition reactions are first order for the studied complexes. To examine the properties of uranyl complexes according to the substitutional groups, we have carried out the electrochemical studies. The electrochemical reactions of uranyl Schiff base complexes in acetonitrile were reversible.
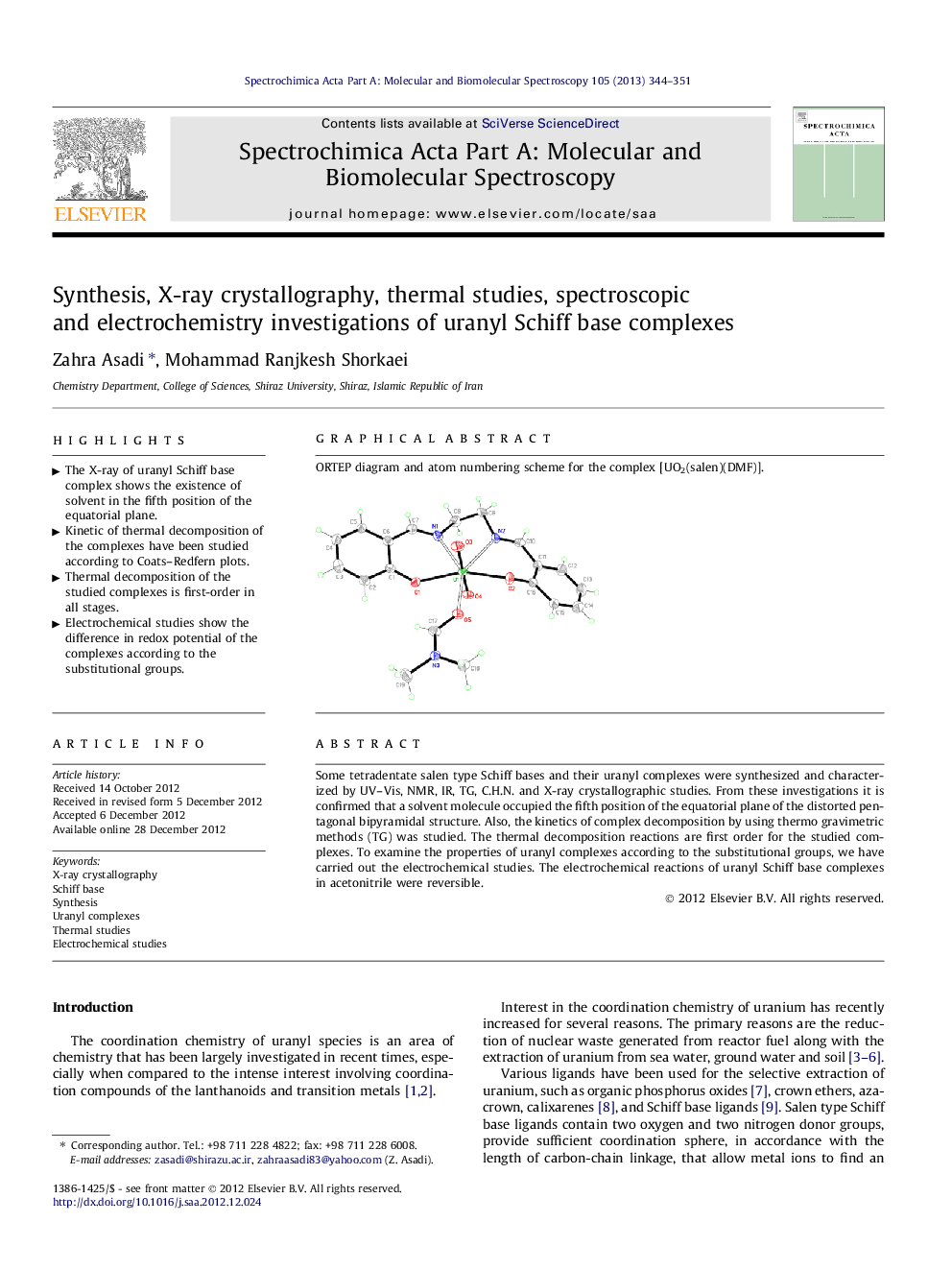
Graphical abstractORTEP diagram and atom numbering scheme for the complex [UO2(salen)(DMF)].Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The X-ray of uranyl Schiff base complex shows the existence of solvent in the fifth position of the equatorial plane. ► Kinetic of thermal decomposition of the complexes have been studied according to Coats–Redfern plots. ► Thermal decomposition of the studied complexes is first-order in all stages. ► Electrochemical studies show the difference in redox potential of the complexes according to the substitutional groups.