| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231904 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
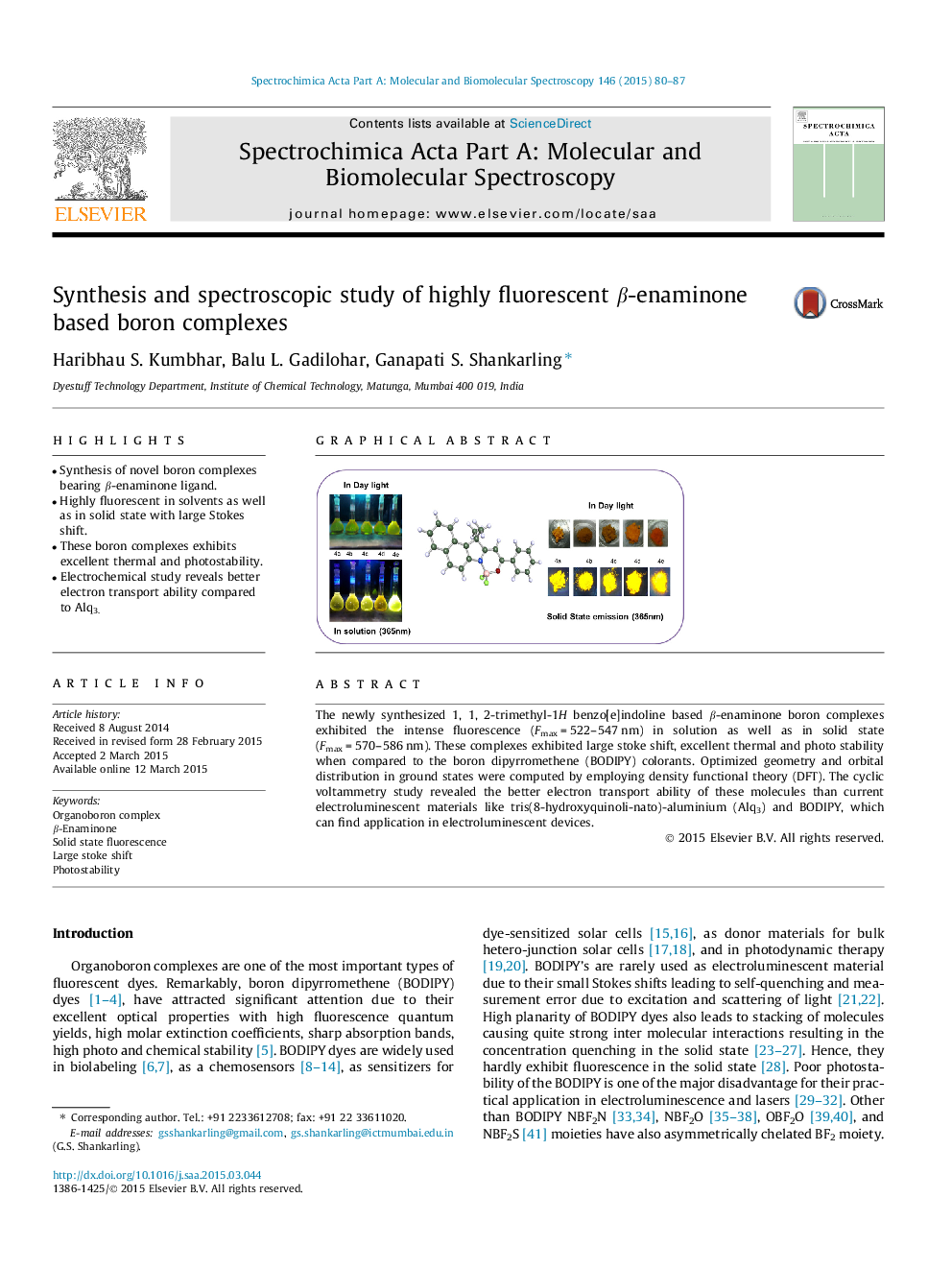
•Synthesis of novel boron complexes bearing β-enaminone ligand.•Highly fluorescent in solvents as well as in solid state with large Stokes shift.•These boron complexes exhibits excellent thermal and photostability.•Electrochemical study reveals better electron transport ability compared to Alq3.
The newly synthesized 1, 1, 2-trimethyl-1H benzo[e]indoline based β-enaminone boron complexes exhibited the intense fluorescence (Fmax = 522–547 nm) in solution as well as in solid state (Fmax = 570–586 nm). These complexes exhibited large stoke shift, excellent thermal and photo stability when compared to the boron dipyrromethene (BODIPY) colorants. Optimized geometry and orbital distribution in ground states were computed by employing density functional theory (DFT). The cyclic voltammetry study revealed the better electron transport ability of these molecules than current electroluminescent materials like tris(8-hydroxyquinoli-nato)-aluminium (Alq3) and BODIPY, which can find application in electroluminescent devices.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide