| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1231912 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 14 Pages |
•Preparation of Co2+, Ni2+ and Cu2+ of hydrazone complexes.•The complexes are characterized by conventional techniques.•Antioxidant and in vitro antitumor activities was tested.
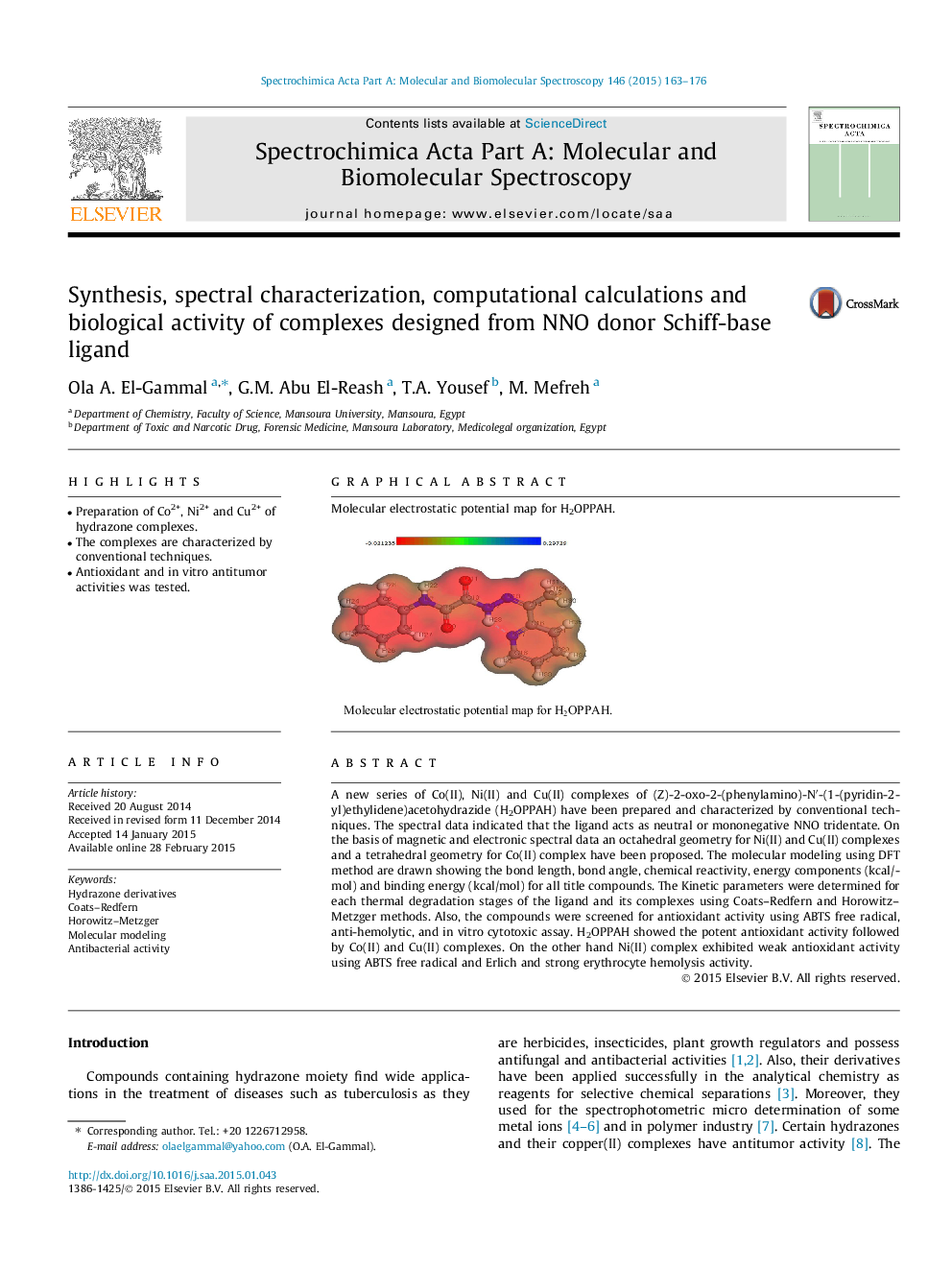
A new series of Co(II), Ni(II) and Cu(II) complexes of (Z)-2-oxo-2-(phenylamino)-N′-(1-(pyridin-2-yl)ethylidene)acetohydrazide (H2OPPAH) have been prepared and characterized by conventional techniques. The spectral data indicated that the ligand acts as neutral or mononegative NNO tridentate. On the basis of magnetic and electronic spectral data an octahedral geometry for Ni(II) and Cu(II) complexes and a tetrahedral geometry for Co(II) complex have been proposed. The molecular modeling using DFT method are drawn showing the bond length, bond angle, chemical reactivity, energy components (kcal/mol) and binding energy (kcal/mol) for all title compounds. The Kinetic parameters were determined for each thermal degradation stages of the ligand and its complexes using Coats–Redfern and Horowitz–Metzger methods. Also, the compounds were screened for antioxidant activity using ABTS free radical, anti-hemolytic, and in vitro cytotoxic assay. H2OPPAH showed the potent antioxidant activity followed by Co(II) and Cu(II) complexes. On the other hand Ni(II) complex exhibited weak antioxidant activity using ABTS free radical and Erlich and strong erythrocyte hemolysis activity.
Graphical abstractMolecular electrostatic potential map for H2OPPAH.Figure optionsDownload full-size imageDownload as PowerPoint slide