| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232033 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
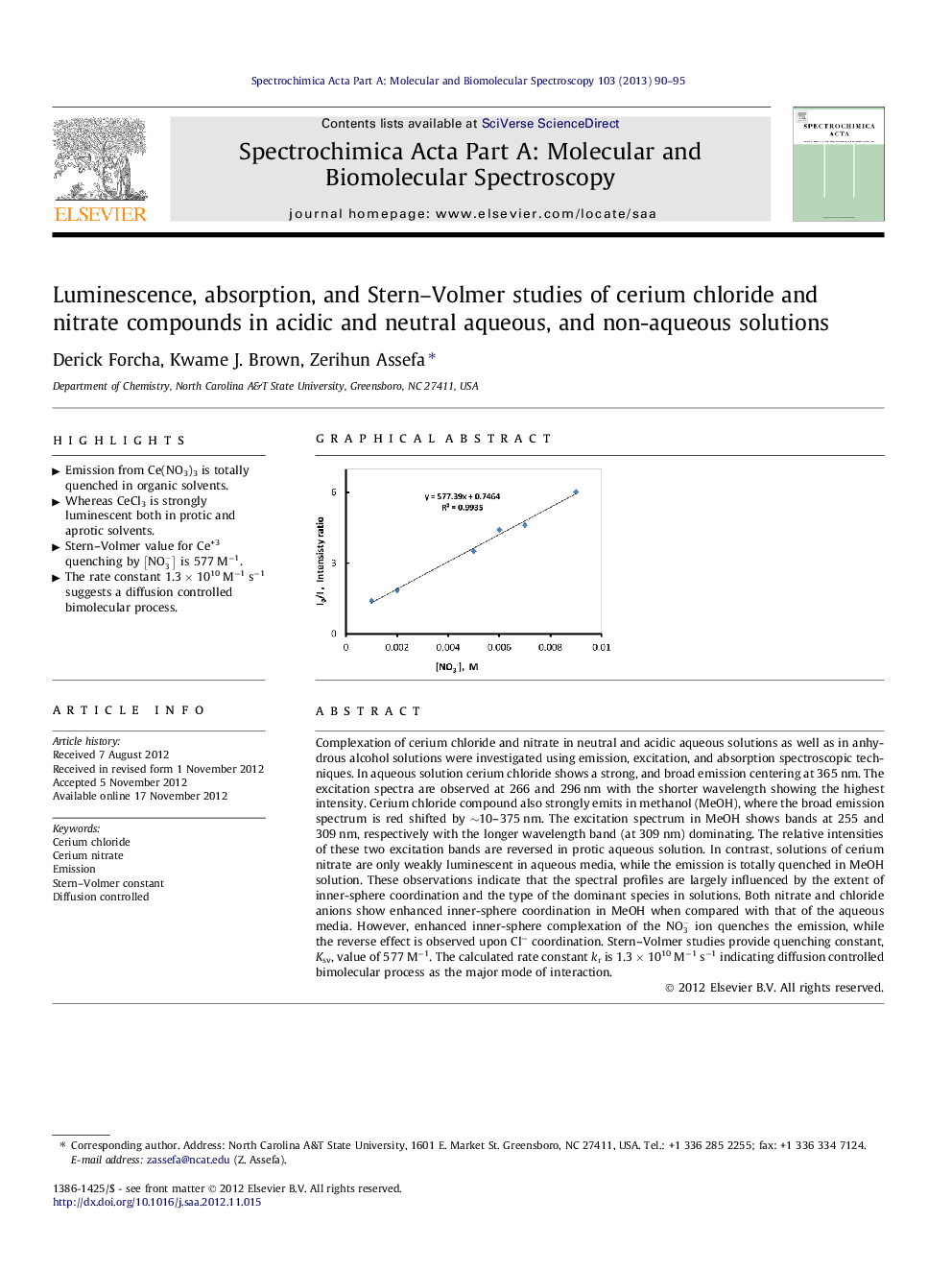
Complexation of cerium chloride and nitrate in neutral and acidic aqueous solutions as well as in anhydrous alcohol solutions were investigated using emission, excitation, and absorption spectroscopic techniques. In aqueous solution cerium chloride shows a strong, and broad emission centering at 365 nm. The excitation spectra are observed at 266 and 296 nm with the shorter wavelength showing the highest intensity. Cerium chloride compound also strongly emits in methanol (MeOH), where the broad emission spectrum is red shifted by ∼10–375 nm. The excitation spectrum in MeOH shows bands at 255 and 309 nm, respectively with the longer wavelength band (at 309 nm) dominating. The relative intensities of these two excitation bands are reversed in protic aqueous solution. In contrast, solutions of cerium nitrate are only weakly luminescent in aqueous media, while the emission is totally quenched in MeOH solution. These observations indicate that the spectral profiles are largely influenced by the extent of inner-sphere coordination and the type of the dominant species in solutions. Both nitrate and chloride anions show enhanced inner-sphere coordination in MeOH when compared with that of the aqueous media. However, enhanced inner-sphere complexation of the NO3- ion quenches the emission, while the reverse effect is observed upon Cl− coordination. Stern–Volmer studies provide quenching constant, Ksv, value of 577 M−1. The calculated rate constant kr is 1.3 × 1010 M−1 s−1 indicating diffusion controlled bimolecular process as the major mode of interaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Emission from Ce(NO3)3 is totally quenched in organic solvents. ► Whereas CeCl3 is strongly luminescent both in protic and aprotic solvents. ► Stern–Volmer value for Ce+3 quenching by NO3- is 577 M−1. ► The rate constant 1.3 × 1010 M−1 s−1 suggests a diffusion controlled bimolecular process.