| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232037 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
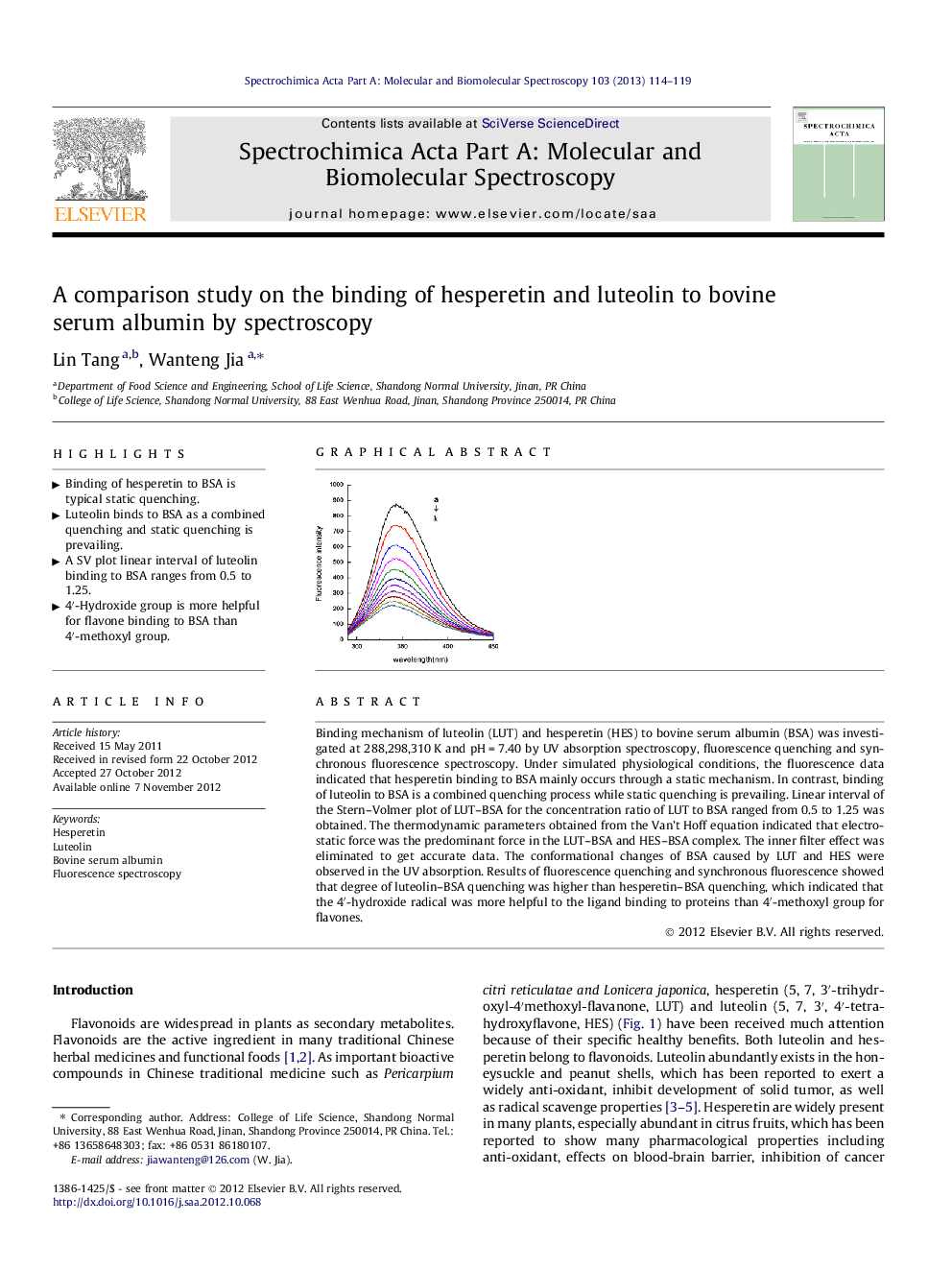
Binding mechanism of luteolin (LUT) and hesperetin (HES) to bovine serum albumin (BSA) was investigated at 288,298,310 K and pH = 7.40 by UV absorption spectroscopy, fluorescence quenching and synchronous fluorescence spectroscopy. Under simulated physiological conditions, the fluorescence data indicated that hesperetin binding to BSA mainly occurs through a static mechanism. In contrast, binding of luteolin to BSA is a combined quenching process while static quenching is prevailing. Linear interval of the Stern–Volmer plot of LUT–BSA for the concentration ratio of LUT to BSA ranged from 0.5 to 1.25 was obtained. The thermodynamic parameters obtained from the Van’t Hoff equation indicated that electrostatic force was the predominant force in the LUT–BSA and HES–BSA complex. The inner filter effect was eliminated to get accurate data. The conformational changes of BSA caused by LUT and HES were observed in the UV absorption. Results of fluorescence quenching and synchronous fluorescence showed that degree of luteolin–BSA quenching was higher than hesperetin–BSA quenching, which indicated that the 4′-hydroxide radical was more helpful to the ligand binding to proteins than 4′-methoxyl group for flavones.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Binding of hesperetin to BSA is typical static quenching. ► Luteolin binds to BSA as a combined quenching and static quenching is prevailing. ► A SV plot linear interval of luteolin binding to BSA ranges from 0.5 to 1.25. ► 4′-Hydroxide group is more helpful for flavone binding to BSA than 4′-methoxyl group.