| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232072 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 7 Pages |
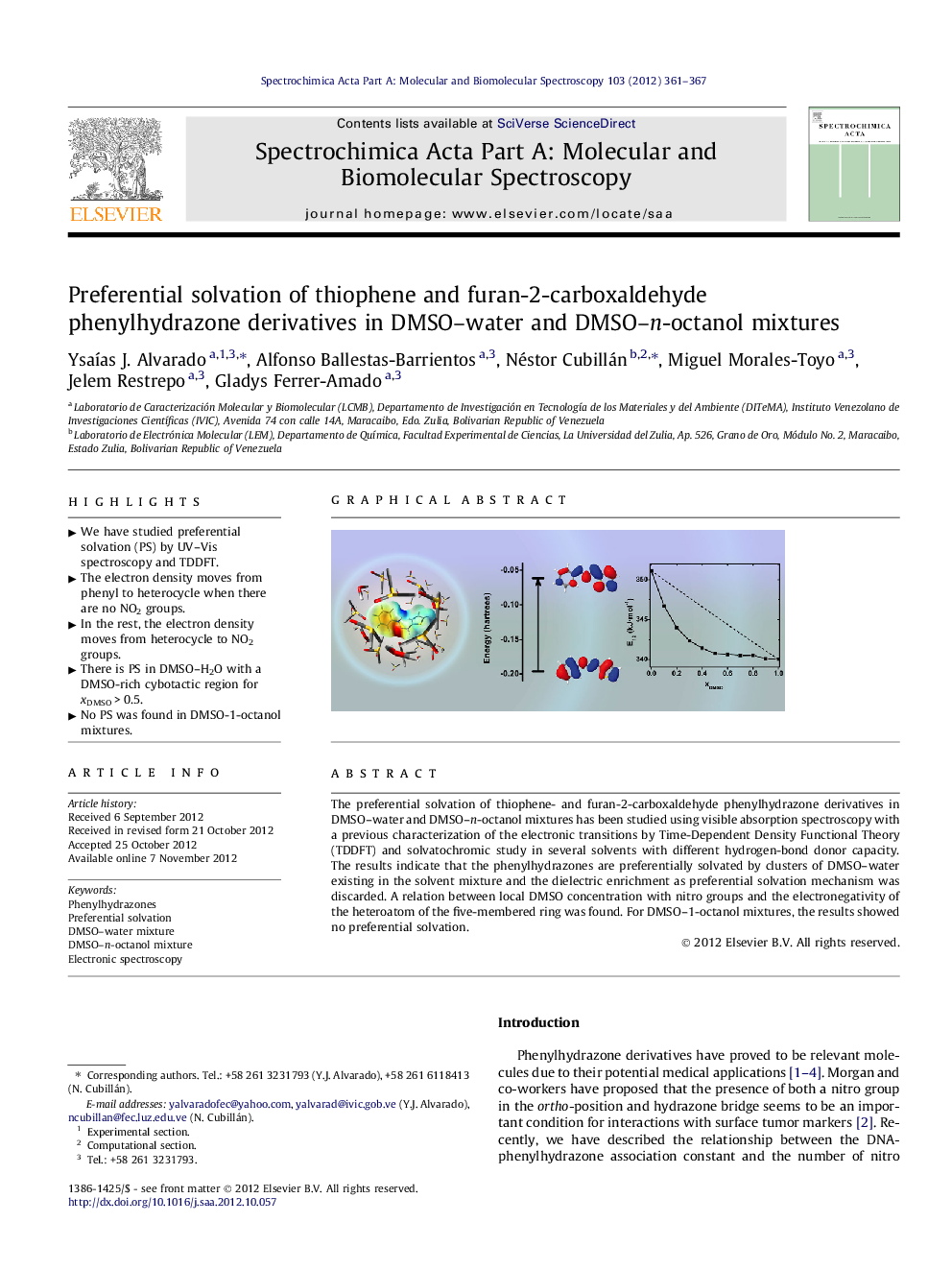
The preferential solvation of thiophene- and furan-2-carboxaldehyde phenylhydrazone derivatives in DMSO–water and DMSO–n-octanol mixtures has been studied using visible absorption spectroscopy with a previous characterization of the electronic transitions by Time-Dependent Density Functional Theory (TDDFT) and solvatochromic study in several solvents with different hydrogen-bond donor capacity. The results indicate that the phenylhydrazones are preferentially solvated by clusters of DMSO–water existing in the solvent mixture and the dielectric enrichment as preferential solvation mechanism was discarded. A relation between local DMSO concentration with nitro groups and the electronegativity of the heteroatom of the five-membered ring was found. For DMSO–1-octanol mixtures, the results showed no preferential solvation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► We have studied preferential solvation (PS) by UV–Vis spectroscopy and TDDFT. ► The electron density moves from phenyl to heterocycle when there are no NO2 groups. ► In the rest, the electron density moves from heterocycle to NO2 groups. ► There is PS in DMSO–H2O with a DMSO-rich cybotactic region for xDMSO > 0.5. ► No PS was found in DMSO-1-octanol mixtures.