| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232092 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
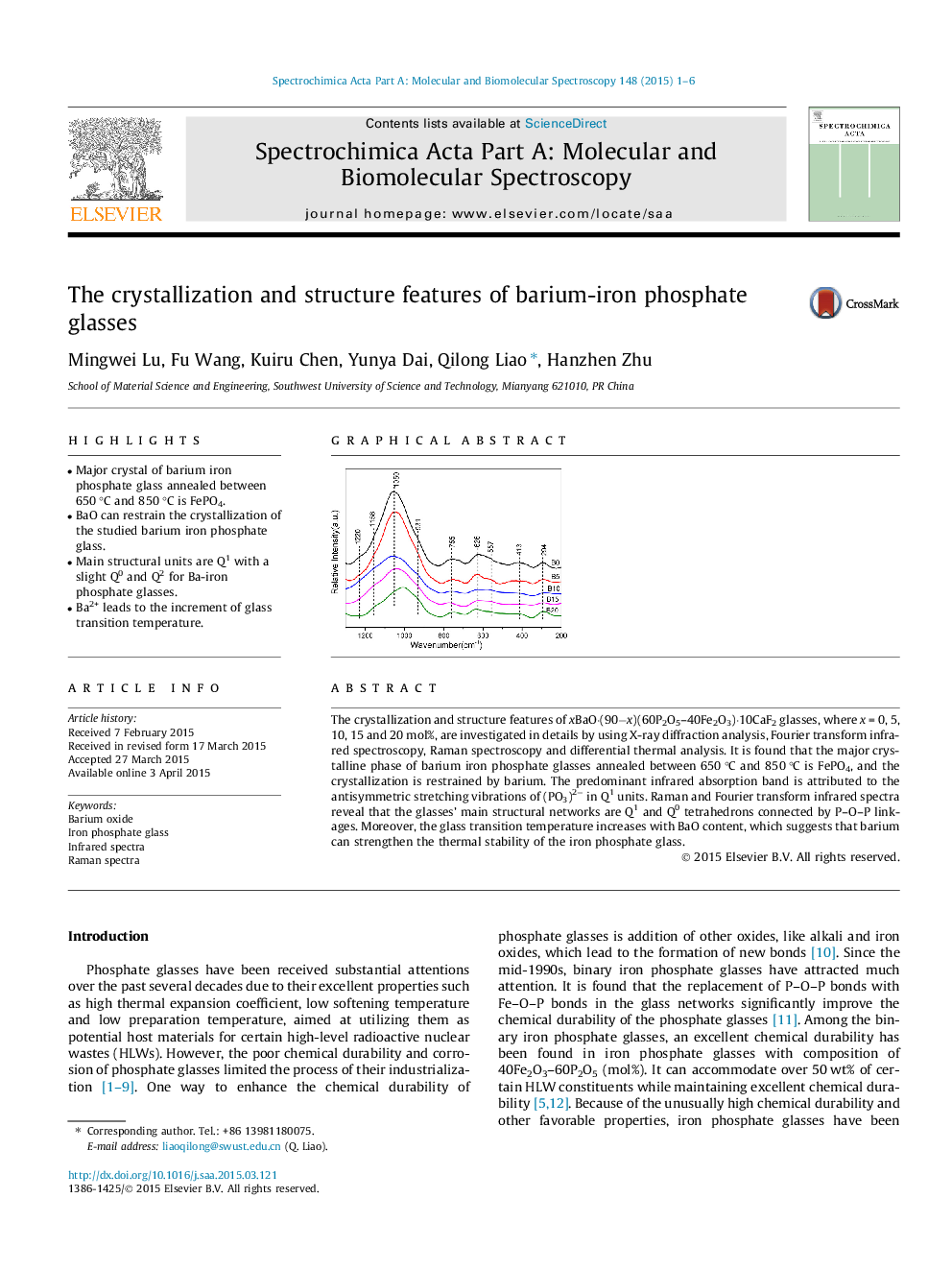
•Major crystal of barium iron phosphate glass annealed between 650 °C and 850 °C is FePO4.•BaO can restrain the crystallization of the studied barium iron phosphate glass.•Main structural units are Q1 with a slight Q0 and Q2 for Ba-iron phosphate glasses.•Ba2+ leads to the increment of glass transition temperature.
The crystallization and structure features of xBaO·(90−x)(60P2O5–40Fe2O3)·10CaF2 glasses, where x = 0, 5, 10, 15 and 20 mol%, are investigated in details by using X-ray diffraction analysis, Fourier transform infrared spectroscopy, Raman spectroscopy and differential thermal analysis. It is found that the major crystalline phase of barium iron phosphate glasses annealed between 650 °C and 850 °C is FePO4, and the crystallization is restrained by barium. The predominant infrared absorption band is attributed to the antisymmetric stretching vibrations of (PO3)2− in Q1 units. Raman and Fourier transform infrared spectra reveal that the glasses’ main structural networks are Q1 and Q0 tetrahedrons connected by P–O–P linkages. Moreover, the glass transition temperature increases with BaO content, which suggests that barium can strengthen the thermal stability of the iron phosphate glass.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide