| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232114 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
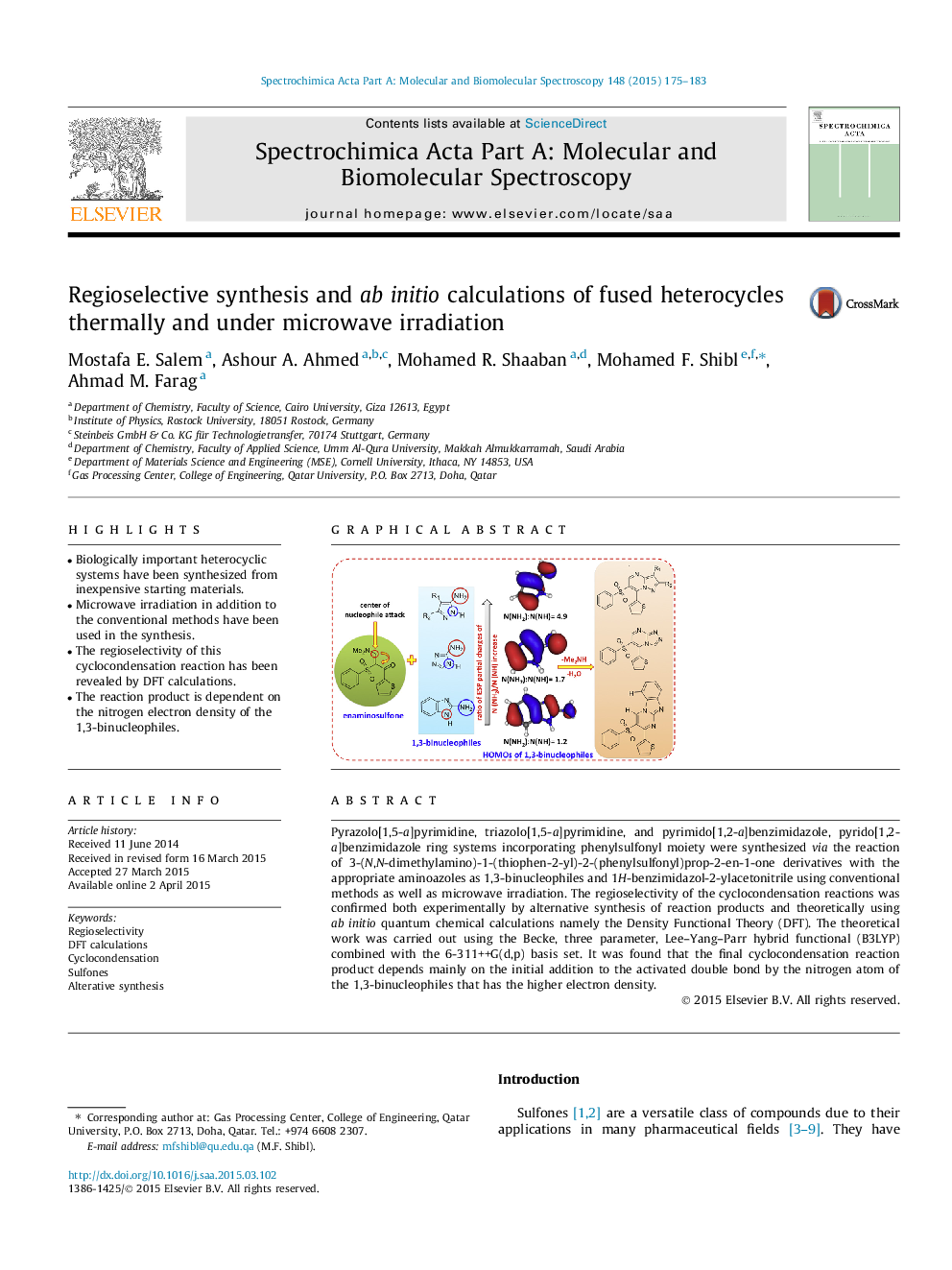
•Biologically important heterocyclic systems have been synthesized from inexpensive starting materials.•Microwave irradiation in addition to the conventional methods have been used in the synthesis.•The regioselectivity of this cyclocondensation reaction has been revealed by DFT calculations.•The reaction product is dependent on the nitrogen electron density of the 1,3-binucleophiles.
Pyrazolo[1,5-a]pyrimidine, triazolo[1,5-a]pyrimidine, and pyrimido[1,2-a]benzimidazole, pyrido[1,2-a]benzimidazole ring systems incorporating phenylsulfonyl moiety were synthesized via the reaction of 3-(N,N-dimethylamino)-1-(thiophen-2-yl)-2-(phenylsulfonyl)prop-2-en-1-one derivatives with the appropriate aminoazoles as 1,3-binucleophiles and 1H-benzimidazol-2-ylacetonitrile using conventional methods as well as microwave irradiation. The regioselectivity of the cyclocondensation reactions was confirmed both experimentally by alternative synthesis of reaction products and theoretically using ab initio quantum chemical calculations namely the Density Functional Theory (DFT). The theoretical work was carried out using the Becke, three parameter, Lee–Yang–Parr hybrid functional (B3LYP) combined with the 6-311++G(d,p) basis set. It was found that the final cyclocondensation reaction product depends mainly on the initial addition to the activated double bond by the nitrogen atom of the 1,3-binucleophiles that has the higher electron density.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide