| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232207 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
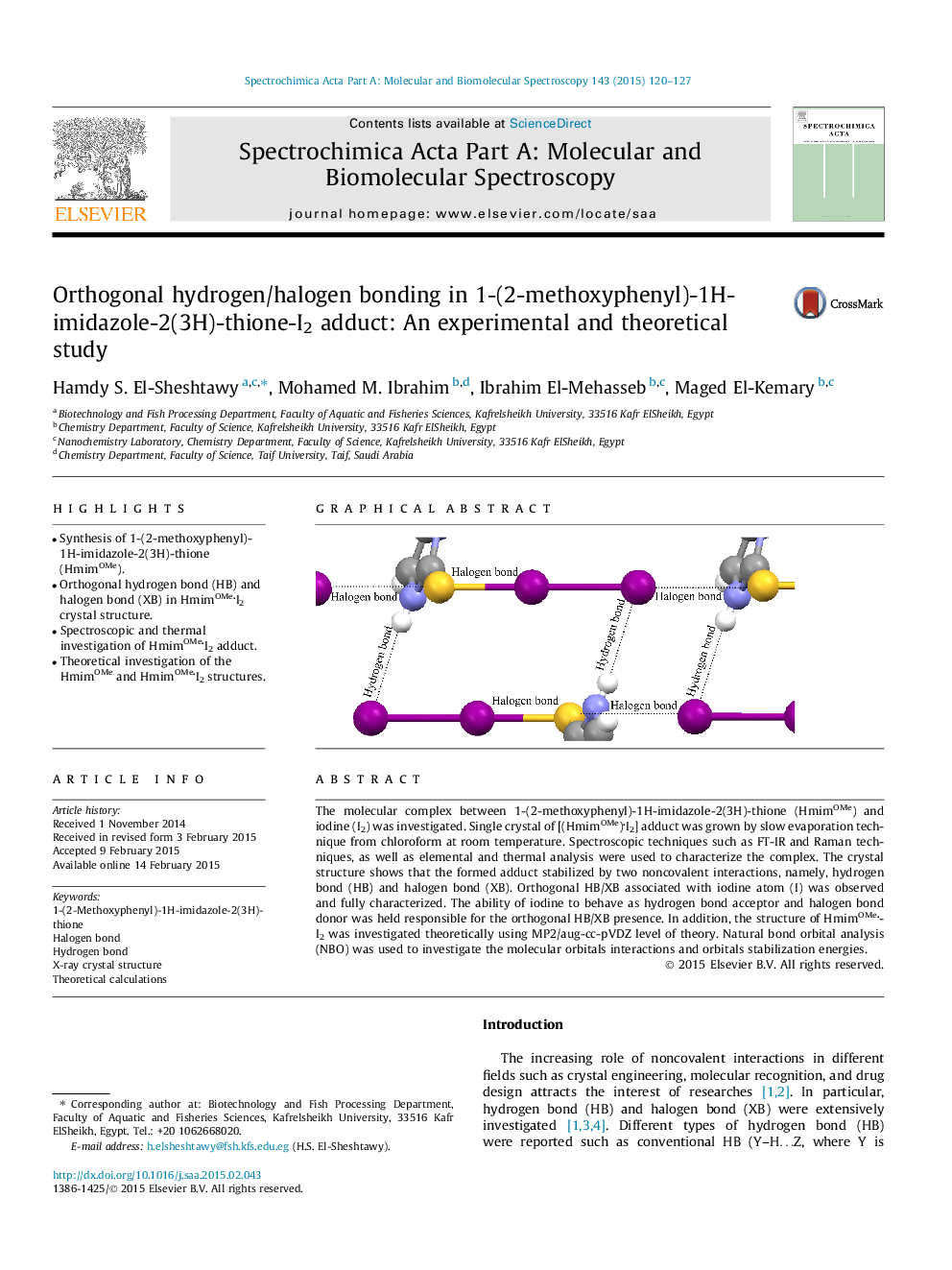
•Synthesis of 1-(2-methoxyphenyl)-1H-imidazole-2(3H)-thione (HmimOMe).•Orthogonal hydrogen bond (HB) and halogen bond (XB) in HmimOMeI2 crystal structure.•Spectroscopic and thermal investigation of HmimOMeI2 adduct.•Theoretical investigation of the HmimOMe and HmimOMeI2 structures.
The molecular complex between 1-(2-methoxyphenyl)-1H-imidazole-2(3H)-thione (HmimOMe) and iodine (I2) was investigated. Single crystal of [(HmimOMe)I2] adduct was grown by slow evaporation technique from chloroform at room temperature. Spectroscopic techniques such as FT-IR and Raman techniques, as well as elemental and thermal analysis were used to characterize the complex. The crystal structure shows that the formed adduct stabilized by two noncovalent interactions, namely, hydrogen bond (HB) and halogen bond (XB). Orthogonal HB/XB associated with iodine atom (I) was observed and fully characterized. The ability of iodine to behave as hydrogen bond acceptor and halogen bond donor was held responsible for the orthogonal HB/XB presence. In addition, the structure of HmimOMeI2 was investigated theoretically using MP2/aug-cc-pVDZ level of theory. Natural bond orbital analysis (NBO) was used to investigate the molecular orbitals interactions and orbitals stabilization energies.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide