| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232215 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
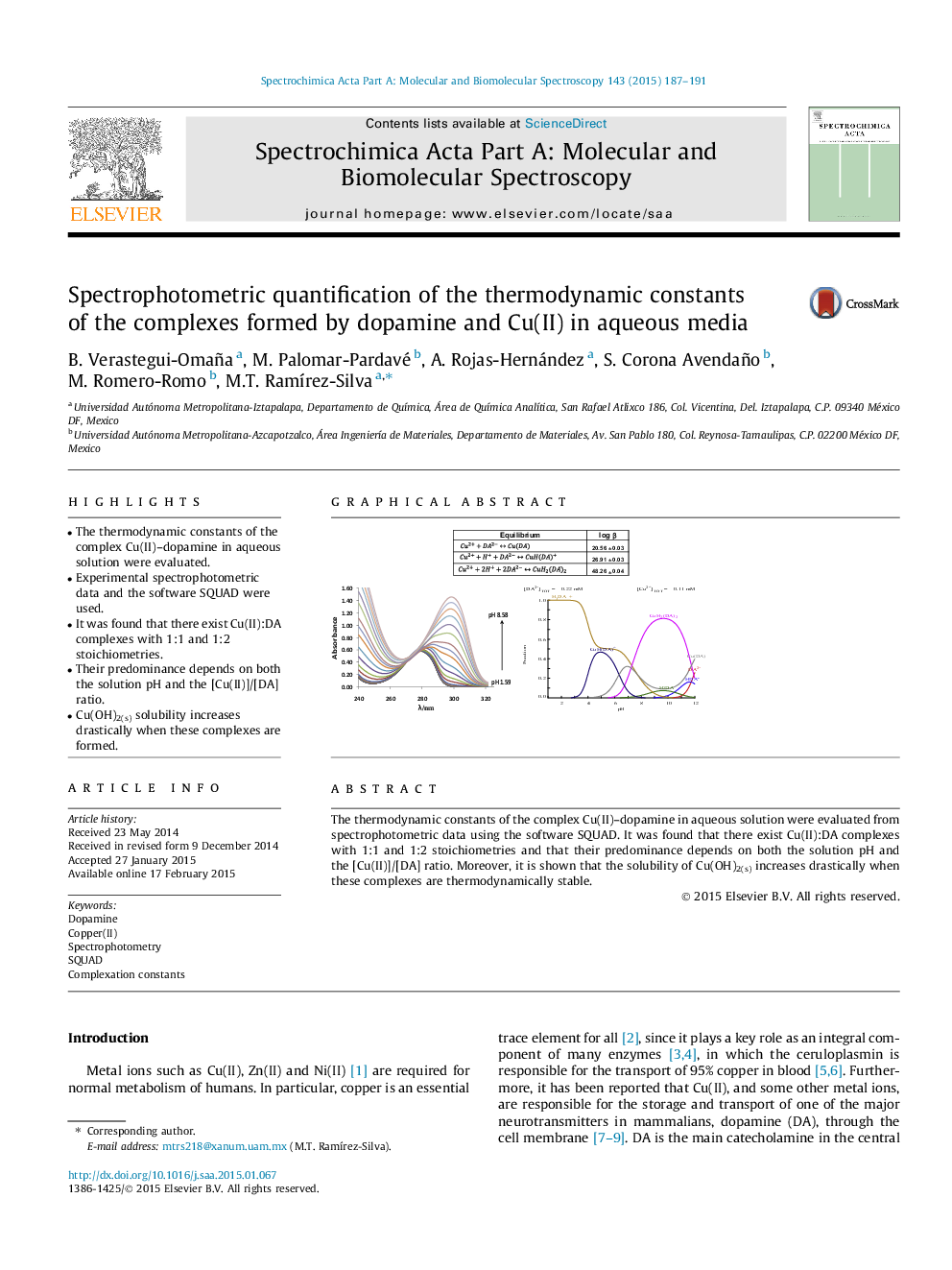
•The thermodynamic constants of the complex Cu(II)–dopamine in aqueous solution were evaluated.•Experimental spectrophotometric data and the software SQUAD were used.•It was found that there exist Cu(II):DA complexes with 1:1 and 1:2 stoichiometries.•Their predominance depends on both the solution pH and the [Cu(II)]/[DA] ratio.•Cu(OH)2(s) solubility increases drastically when these complexes are formed.
The thermodynamic constants of the complex Cu(II)–dopamine in aqueous solution were evaluated from spectrophotometric data using the software SQUAD. It was found that there exist Cu(II):DA complexes with 1:1 and 1:2 stoichiometries and that their predominance depends on both the solution pH and the [Cu(II)]/[DA] ratio. Moreover, it is shown that the solubility of Cu(OH)2(s) increases drastically when these complexes are thermodynamically stable.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide