| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232341 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 5 Pages |
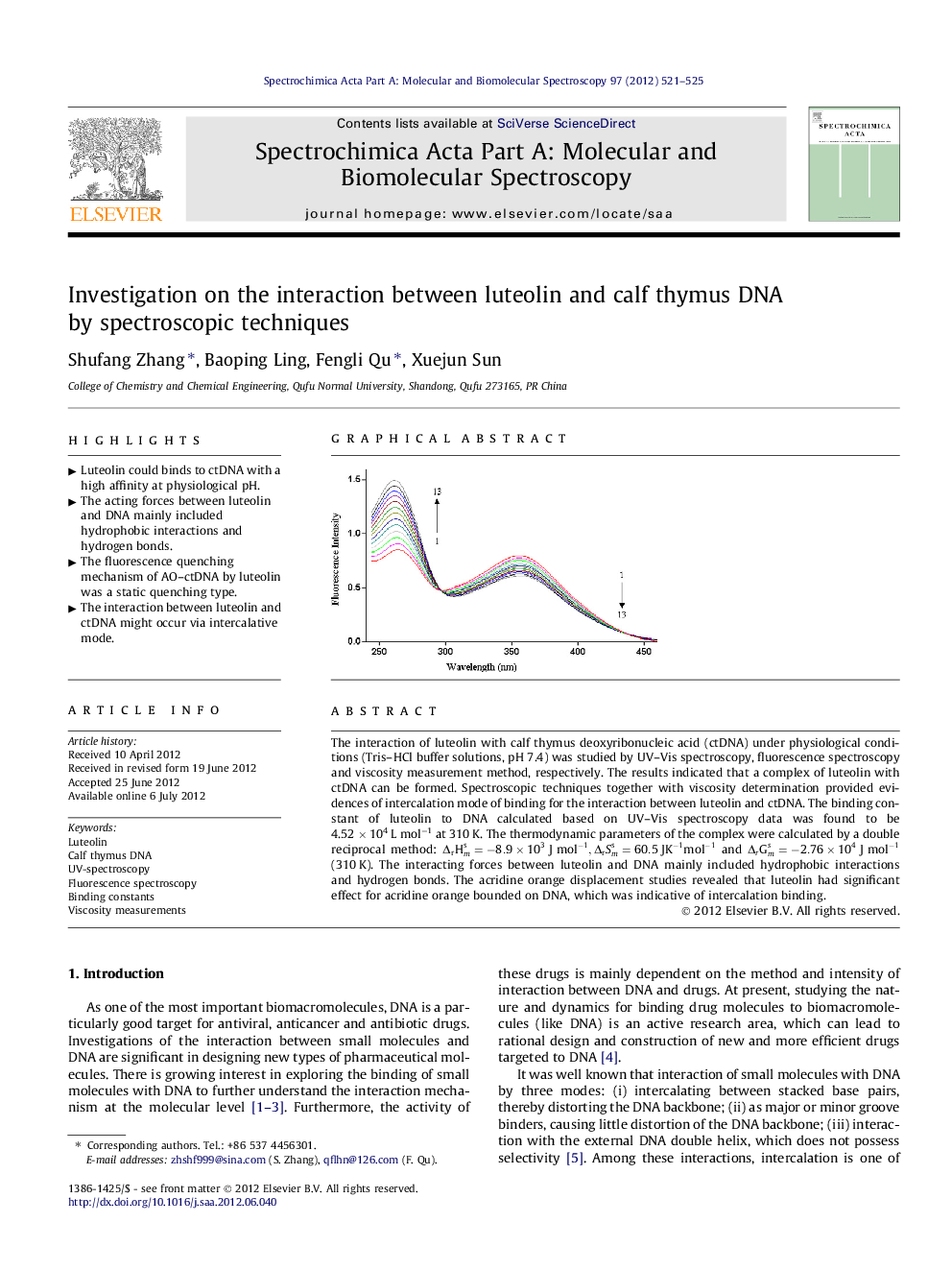
The interaction of luteolin with calf thymus deoxyribonucleic acid (ctDNA) under physiological conditions (Tris–HCl buffer solutions, pH 7.4) was studied by UV–Vis spectroscopy, fluorescence spectroscopy and viscosity measurement method, respectively. The results indicated that a complex of luteolin with ctDNA can be formed. Spectroscopic techniques together with viscosity determination provided evidences of intercalation mode of binding for the interaction between luteolin and ctDNA. The binding constant of luteolin to DNA calculated based on UV–Vis spectroscopy data was found to be 4.52 × 104 L mol−1 at 310 K. The thermodynamic parameters of the complex were calculated by a double reciprocal method: ΔrHms=-8.9×103J mol-1,ΔrSms=60.5JK-1mol-1 and ΔrGms=-2.76×104J mol-1 (310 K). The interacting forces between luteolin and DNA mainly included hydrophobic interactions and hydrogen bonds. The acridine orange displacement studies revealed that luteolin had significant effect for acridine orange bounded on DNA, which was indicative of intercalation binding.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Luteolin could binds to ctDNA with a high affinity at physiological pH. ► The acting forces between luteolin and DNA mainly included hydrophobic interactions and hydrogen bonds. ► The fluorescence quenching mechanism of AO–ctDNA by luteolin was a static quenching type. ► The interaction between luteolin and ctDNA might occur via intercalative mode.