| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232347 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 7 Pages |
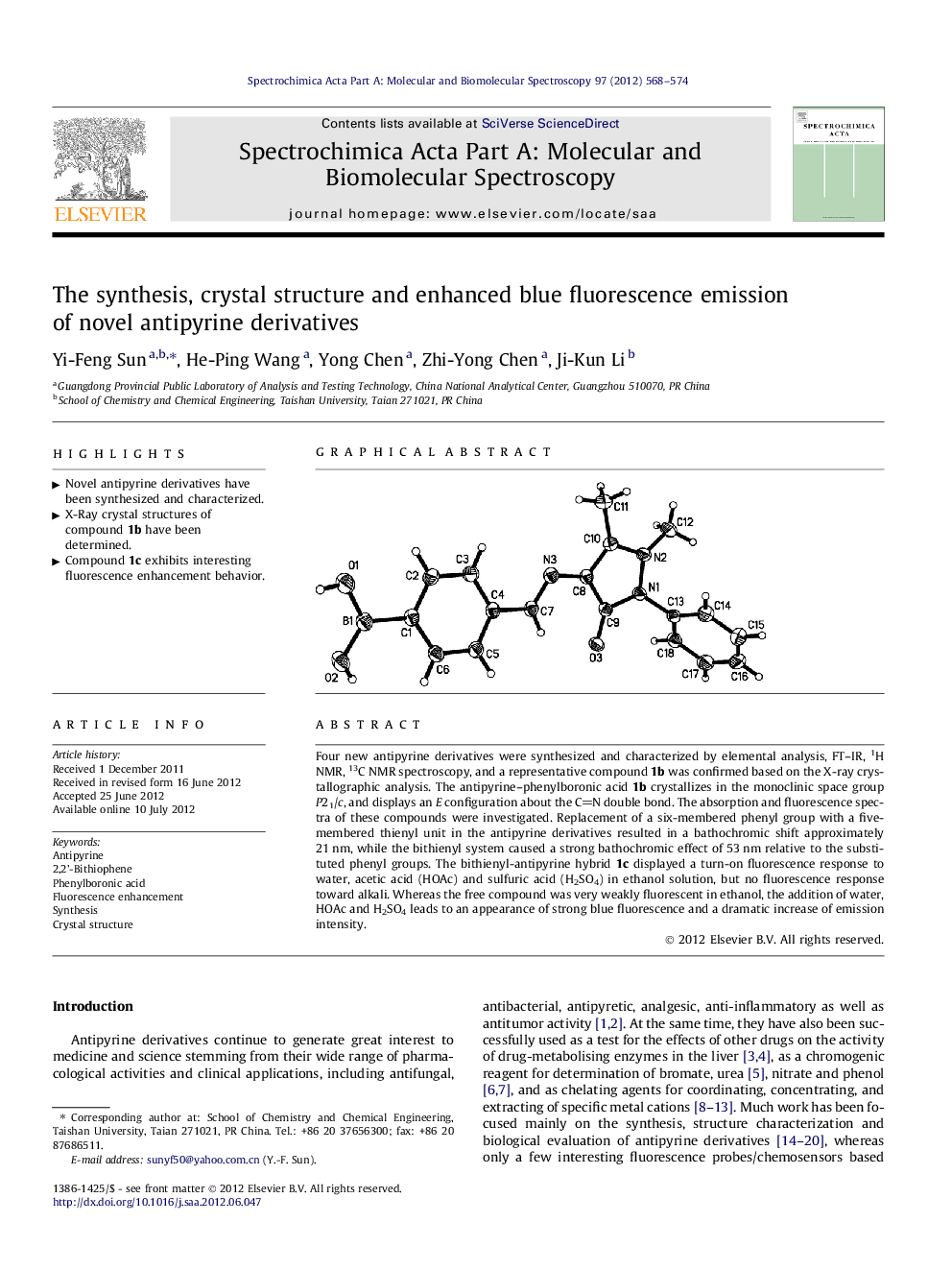
Four new antipyrine derivatives were synthesized and characterized by elemental analysis, FT–IR, 1H NMR, 13C NMR spectroscopy, and a representative compound 1b was confirmed based on the X-ray crystallographic analysis. The antipyrine–phenylboronic acid 1b crystallizes in the monoclinic space group P21/c, and displays an E configuration about the CN double bond. The absorption and fluorescence spectra of these compounds were investigated. Replacement of a six-membered phenyl group with a five-membered thienyl unit in the antipyrine derivatives resulted in a bathochromic shift approximately 21 nm, while the bithienyl system caused a strong bathochromic effect of 53 nm relative to the substituted phenyl groups. The bithienyl-antipyrine hybrid 1c displayed a turn-on fluorescence response to water, acetic acid (HOAc) and sulfuric acid (H2SO4) in ethanol solution, but no fluorescence response toward alkali. Whereas the free compound was very weakly fluorescent in ethanol, the addition of water, HOAc and H2SO4 leads to an appearance of strong blue fluorescence and a dramatic increase of emission intensity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Novel antipyrine derivatives have been synthesized and characterized. ► X-Ray crystal structures of compound 1b have been determined. ► Compound 1c exhibits interesting fluorescence enhancement behavior.