| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232386 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
•Effects of halogen and solvent on conformer and CO stretching of CFB, CCB and CBB.•Carbonyl vibrations were correlated with the KBM, AN, Swain and LSER equations.•DFT method was used to predict structural and vibrational parameters.•Conformational energy barrier is independent of the solvent for CFB.•Theoretical frequencies exhibit good linear correlations with the KBM.
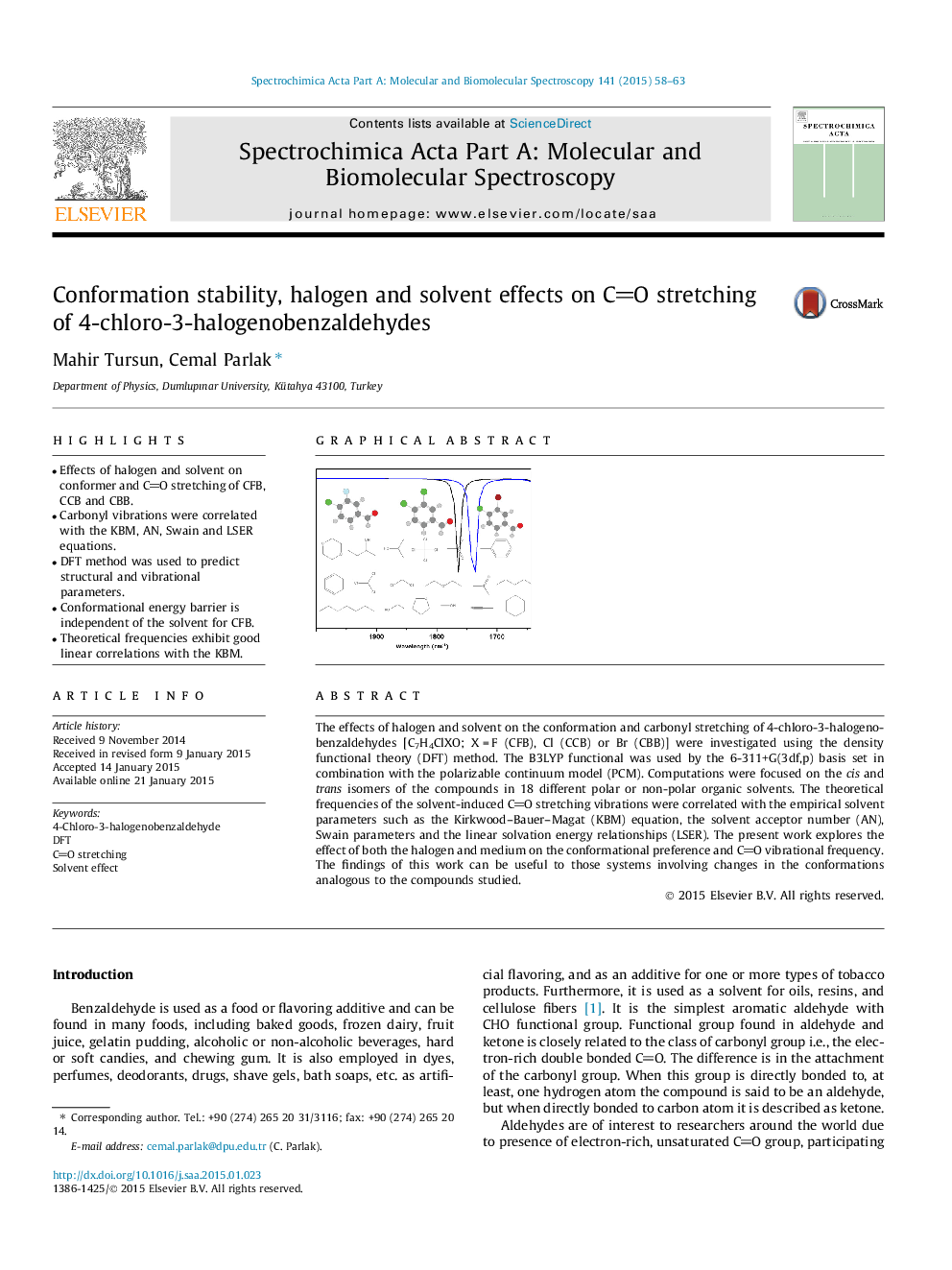
The effects of halogen and solvent on the conformation and carbonyl stretching of 4-chloro-3-halogenobenzaldehydes [C7H4ClXO; X = F (CFB), Cl (CCB) or Br (CBB)] were investigated using the density functional theory (DFT) method. The B3LYP functional was used by the 6-311+G(3df,p) basis set in combination with the polarizable continuum model (PCM). Computations were focused on the cis and trans isomers of the compounds in 18 different polar or non-polar organic solvents. The theoretical frequencies of the solvent-induced CO stretching vibrations were correlated with the empirical solvent parameters such as the Kirkwood–Bauer–Magat (KBM) equation, the solvent acceptor number (AN), Swain parameters and the linear solvation energy relationships (LSER). The present work explores the effect of both the halogen and medium on the conformational preference and CO vibrational frequency. The findings of this work can be useful to those systems involving changes in the conformations analogous to the compounds studied.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide