| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232388 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
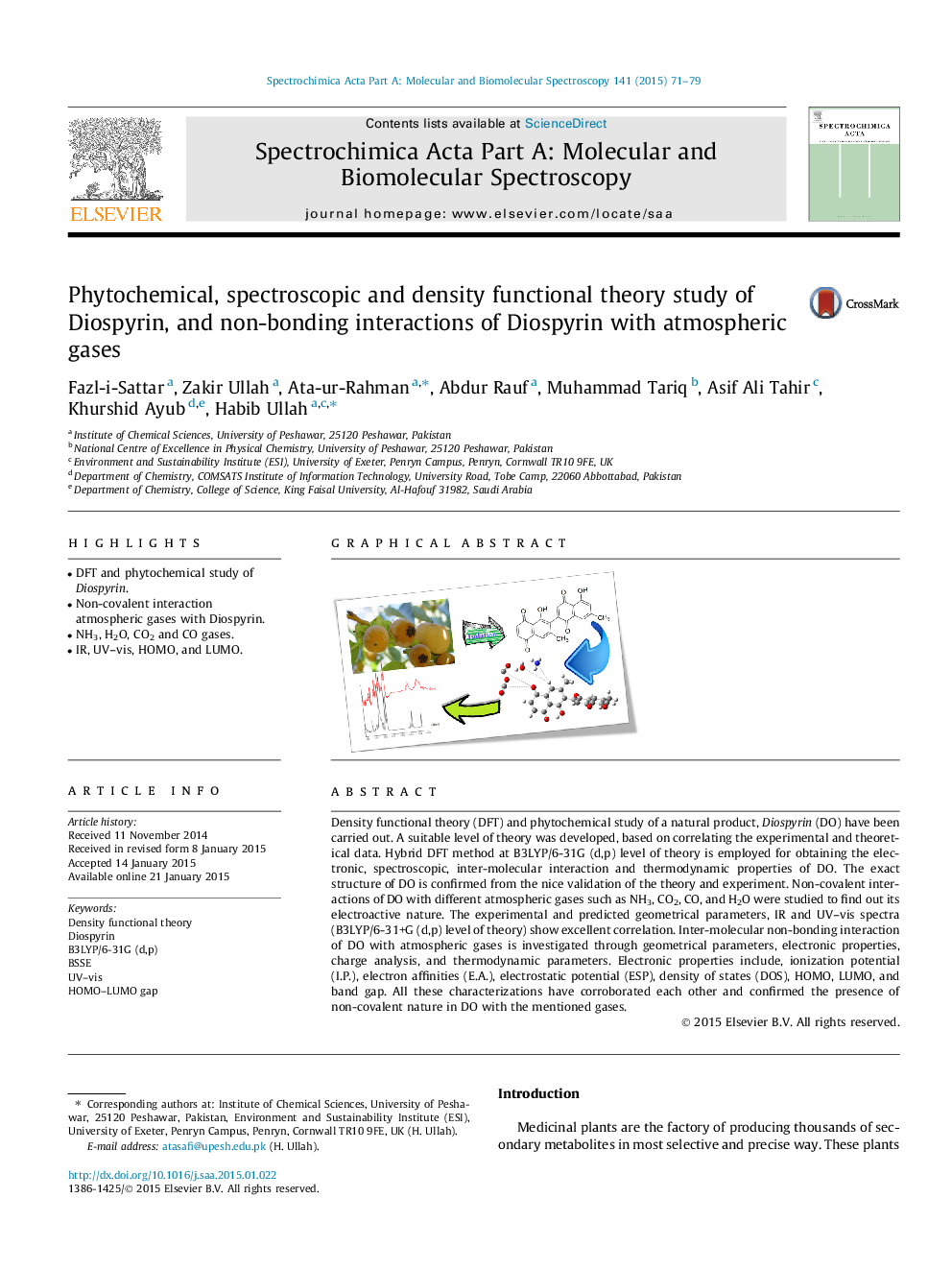
•DFT and phytochemical study of Diospyrin.•Non-covalent interaction atmospheric gases with Diospyrin.•NH3, H2O, CO2 and CO gases.•IR, UV–vis, HOMO, and LUMO.
Density functional theory (DFT) and phytochemical study of a natural product, Diospyrin (DO) have been carried out. A suitable level of theory was developed, based on correlating the experimental and theoretical data. Hybrid DFT method at B3LYP/6-31G (d,p) level of theory is employed for obtaining the electronic, spectroscopic, inter-molecular interaction and thermodynamic properties of DO. The exact structure of DO is confirmed from the nice validation of the theory and experiment. Non-covalent interactions of DO with different atmospheric gases such as NH3, CO2, CO, and H2O were studied to find out its electroactive nature. The experimental and predicted geometrical parameters, IR and UV–vis spectra (B3LYP/6-31+G (d,p) level of theory) show excellent correlation. Inter-molecular non-bonding interaction of DO with atmospheric gases is investigated through geometrical parameters, electronic properties, charge analysis, and thermodynamic parameters. Electronic properties include, ionization potential (I.P.), electron affinities (E.A.), electrostatic potential (ESP), density of states (DOS), HOMO, LUMO, and band gap. All these characterizations have corroborated each other and confirmed the presence of non-covalent nature in DO with the mentioned gases.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide