| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232403 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
•No kinetic study has been reported for the gelatin–CdS direct conjugate.•The nonlinear fitting and the differential method were used to obtain the initial rate.•A double-log linear equation introduced can calculate the rate constant and the reaction order.•The rate equation has been established as a function of reactant concentration and temperature.
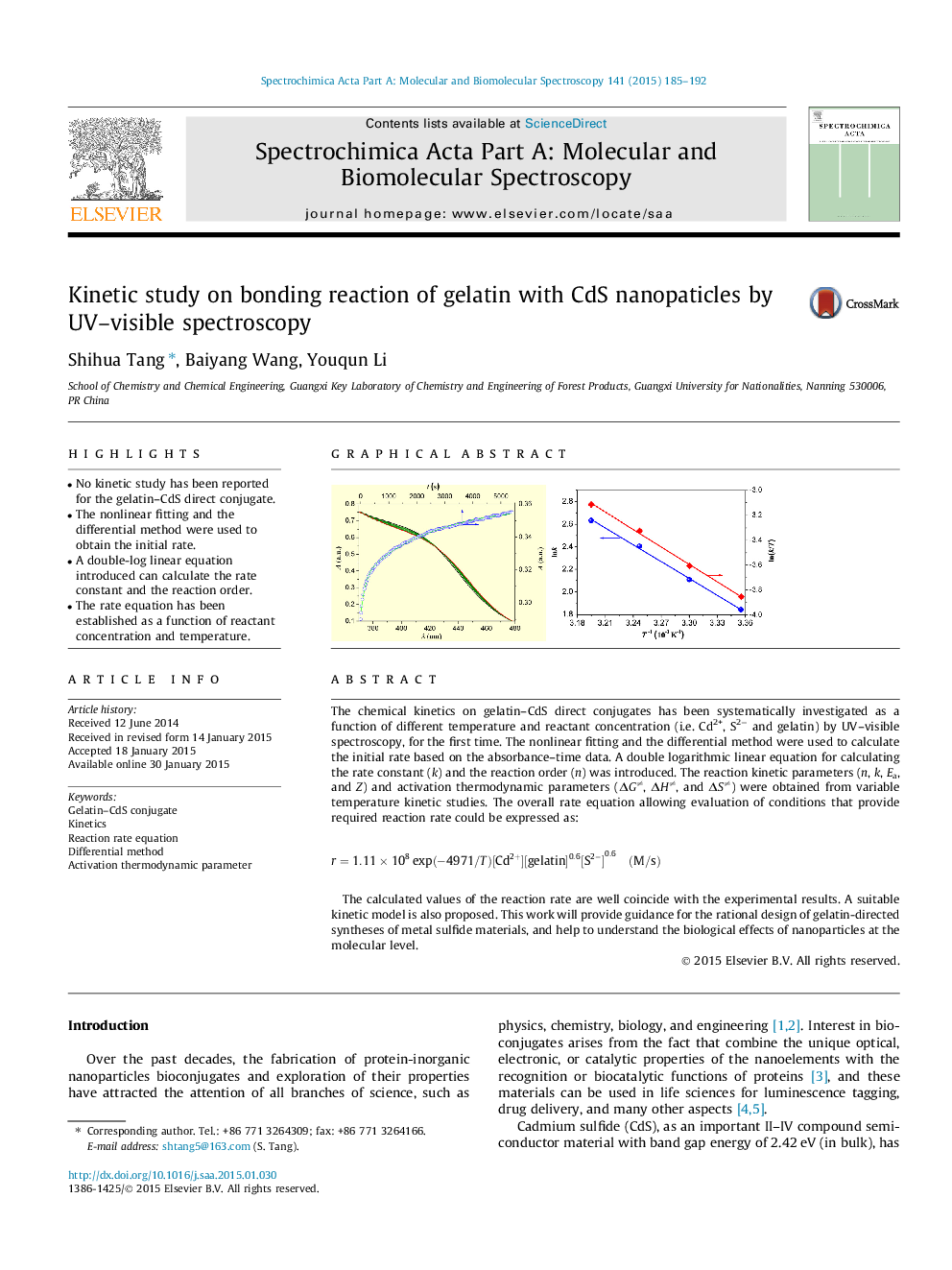
The chemical kinetics on gelatin–CdS direct conjugates has been systematically investigated as a function of different temperature and reactant concentration (i.e. Cd2+, S2− and gelatin) by UV–visible spectroscopy, for the first time. The nonlinear fitting and the differential method were used to calculate the initial rate based on the absorbance–time data. A double logarithmic linear equation for calculating the rate constant (k) and the reaction order (n) was introduced. The reaction kinetic parameters (n, k, Ea, and Z) and activation thermodynamic parameters (ΔG≠, ΔH≠, and ΔS≠) were obtained from variable temperature kinetic studies. The overall rate equation allowing evaluation of conditions that provide required reaction rate could be expressed as:r=1.11×108exp(-4971/T)[Cd2+][gelatin]0.6[S2-]0.6(M/s)The calculated values of the reaction rate are well coincide with the experimental results. A suitable kinetic model is also proposed. This work will provide guidance for the rational design of gelatin-directed syntheses of metal sulfide materials, and help to understand the biological effects of nanoparticles at the molecular level.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide