| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232408 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•Schiff base complexes derived from condensation of 3-acetylcoumarine and diethylenetriamine were synthesized.•The complexes are characterized by different spectroscopic techniques.•The complexes have different varieties of geometrical structures.•Biochemical studies were performed.
Two series of new mono and binuclear complexes with a Schiff base ligand derived from the condensation of 3-acetylcoumarine and diethylenetriamine, in the molar ratio 2:1 have been prepared. The ligand was characterized by elemental analysis, IR, UV–visible, 1H-NMR and mass spectra. The reaction of the Schiff base ligand with cobalt(II), nickel(II), copper(II), zinc(II) and oxovanadium(IV) lead to mono or binuclear species of cyclic or macrocyclic complexes, depending on the mole ratio of metal to ligand and as well as on the method of preparation. The Schiff base ligand behaves as a cyclic bidentate, tetradendate or pentaentadentae ligand. The formation of macrocyclic complexes depends significantly on the dimension of the internal cavity, the rigidity of the macrocycles, the nature of its donor atoms and on the complexing properties of the anion involved in the coordination. Electronic spectra and magnetic moments of the complexes indicate that the geometries of the metal centers are either square pyramidal or octahedral for acyclic or macro-cyclic complexes. The structures are consistent with the IR, UV–visible, ESR, 1H-NMR, mass spectra as well as conductivity and magnetic moment measurements. The Schiff base ligand and its metal complexes were tested against two pathogenic bacteria as Gram-positive and Gram-negative bacteria as well as one kind of fungi. Most of the complexes exhibit mild antibacterial and antifungal activities against these organisms.
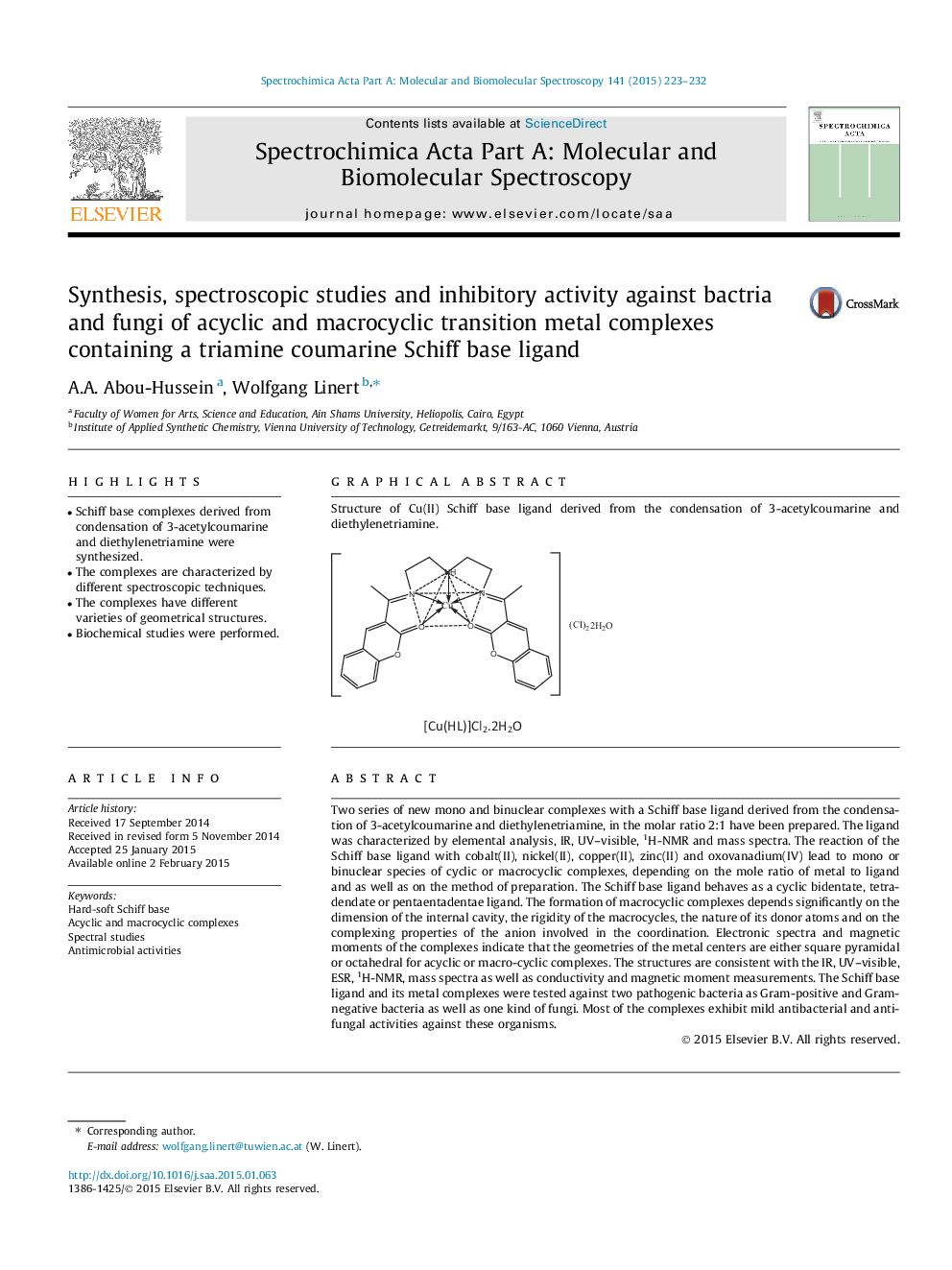
Graphical abstractStructure of Cu(II) Schiff base ligand derived from the condensation of 3-acetylcoumarine and diethylenetriamine.Figure optionsDownload full-size imageDownload as PowerPoint slide