| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232432 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
•Silver nano-particles were synthesized by a green procedure.•Aqueous extract of Eucalyptus oleosa was used to synthesis of silver nano-particles.•Taguchi robust design was used for optimization of synthesis procedure parameters.•Silver nano-particles were characterized by means of UV–Vis, EDX, SEM, and FT-IR.
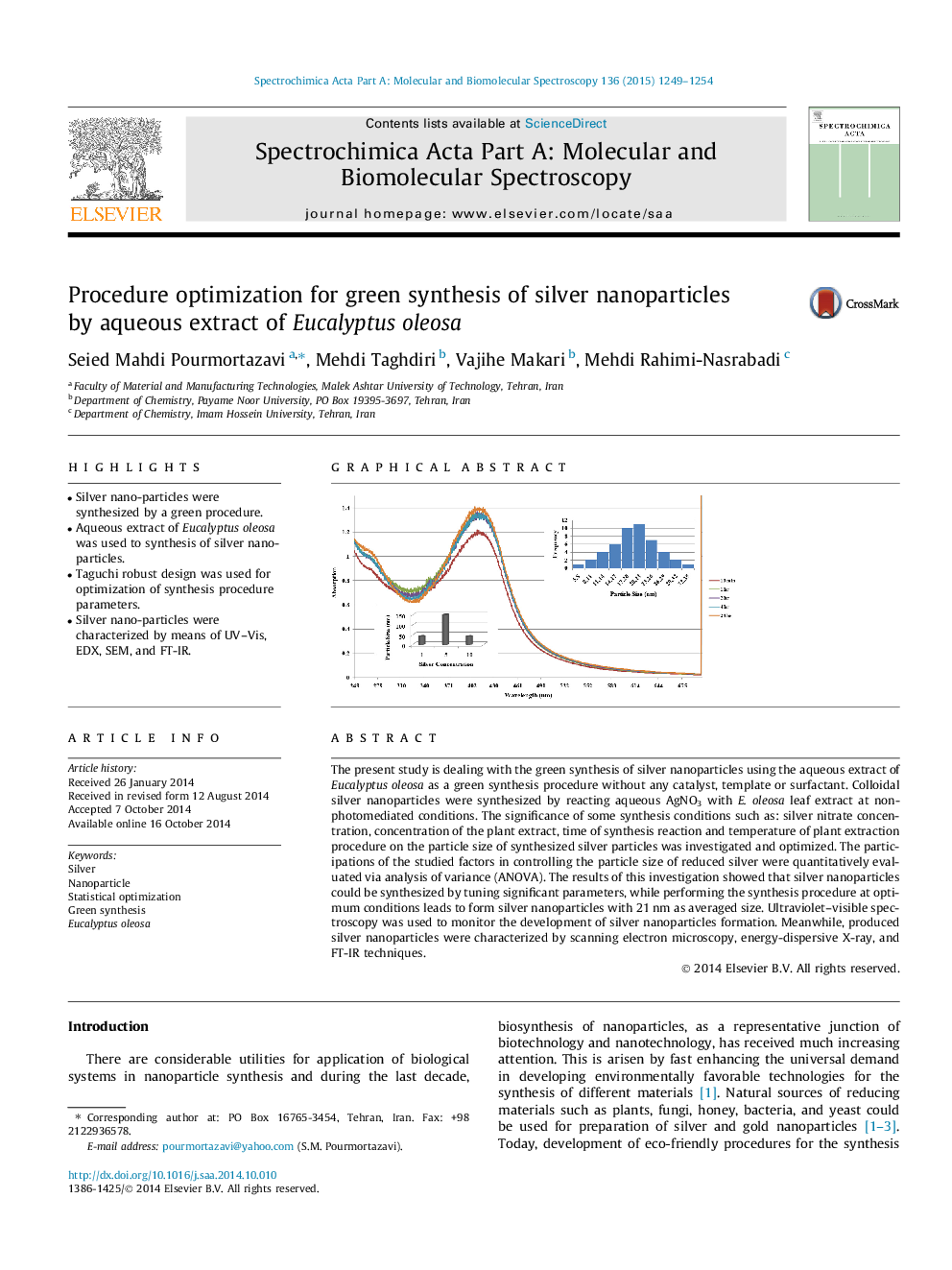
The present study is dealing with the green synthesis of silver nanoparticles using the aqueous extract of Eucalyptus oleosa as a green synthesis procedure without any catalyst, template or surfactant. Colloidal silver nanoparticles were synthesized by reacting aqueous AgNO3 with E. oleosa leaf extract at non-photomediated conditions. The significance of some synthesis conditions such as: silver nitrate concentration, concentration of the plant extract, time of synthesis reaction and temperature of plant extraction procedure on the particle size of synthesized silver particles was investigated and optimized. The participations of the studied factors in controlling the particle size of reduced silver were quantitatively evaluated via analysis of variance (ANOVA). The results of this investigation showed that silver nanoparticles could be synthesized by tuning significant parameters, while performing the synthesis procedure at optimum conditions leads to form silver nanoparticles with 21 nm as averaged size. Ultraviolet–visible spectroscopy was used to monitor the development of silver nanoparticles formation. Meanwhile, produced silver nanoparticles were characterized by scanning electron microscopy, energy-dispersive X-ray, and FT-IR techniques.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide