| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232444 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 13 Pages |
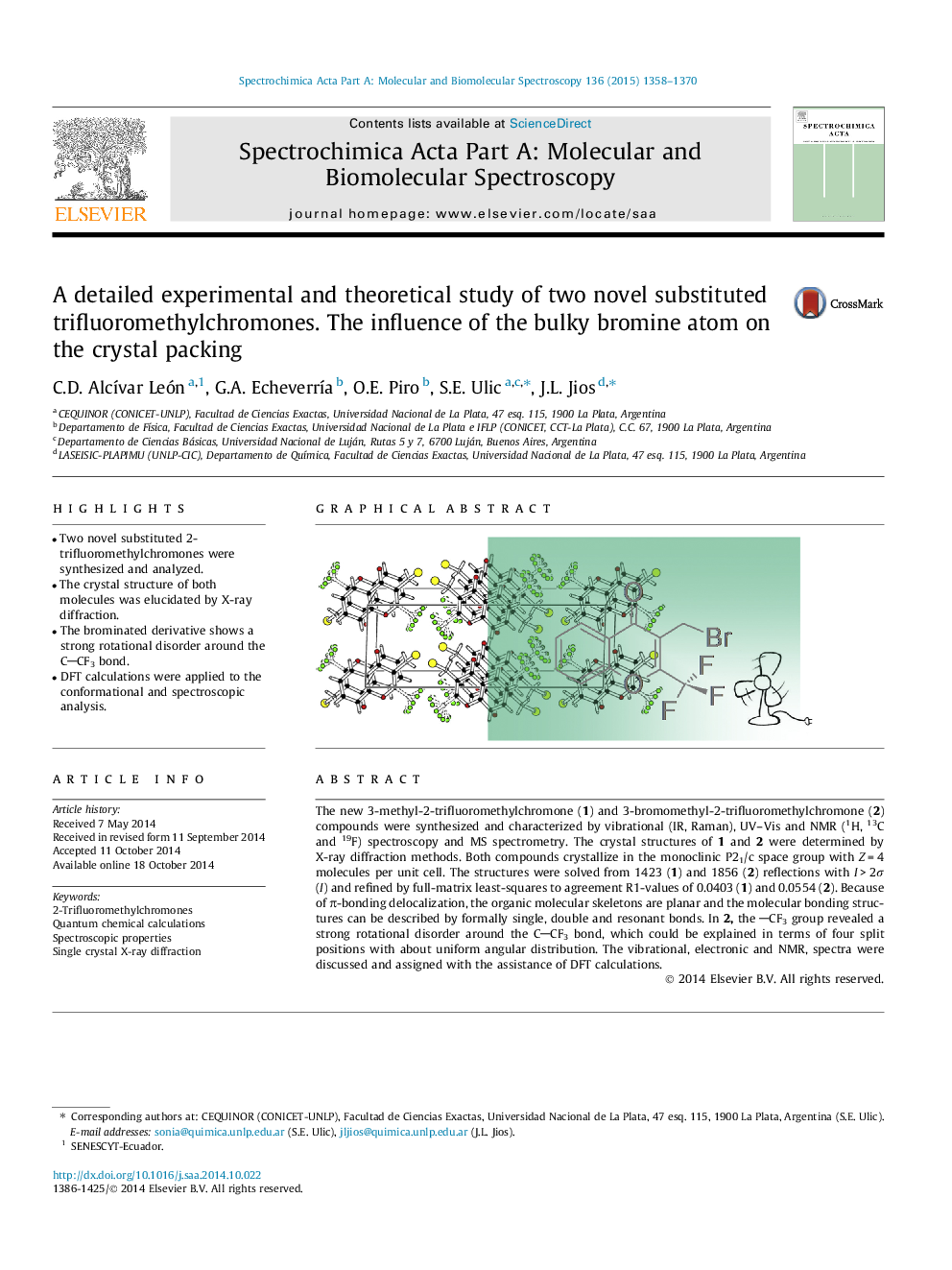
•Two novel substituted 2-trifluoromethylchromones were synthesized and analyzed.•The crystal structure of both molecules was elucidated by X-ray diffraction.•The brominated derivative shows a strong rotational disorder around the CCF3 bond.•DFT calculations were applied to the conformational and spectroscopic analysis.
The new 3-methyl-2-trifluoromethylchromone (1) and 3-bromomethyl-2-trifluoromethylchromone (2) compounds were synthesized and characterized by vibrational (IR, Raman), UV–Vis and NMR (1H, 13C and 19F) spectroscopy and MS spectrometry. The crystal structures of 1 and 2 were determined by X-ray diffraction methods. Both compounds crystallize in the monoclinic P21/c space group with Z = 4 molecules per unit cell. The structures were solved from 1423 (1) and 1856 (2) reflections with I > 2σ (I) and refined by full-matrix least-squares to agreement R1-values of 0.0403 (1) and 0.0554 (2). Because of π-bonding delocalization, the organic molecular skeletons are planar and the molecular bonding structures can be described by formally single, double and resonant bonds. In 2, the CF3 group revealed a strong rotational disorder around the CCF3 bond, which could be explained in terms of four split positions with about uniform angular distribution. The vibrational, electronic and NMR, spectra were discussed and assigned with the assistance of DFT calculations.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide