| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232470 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
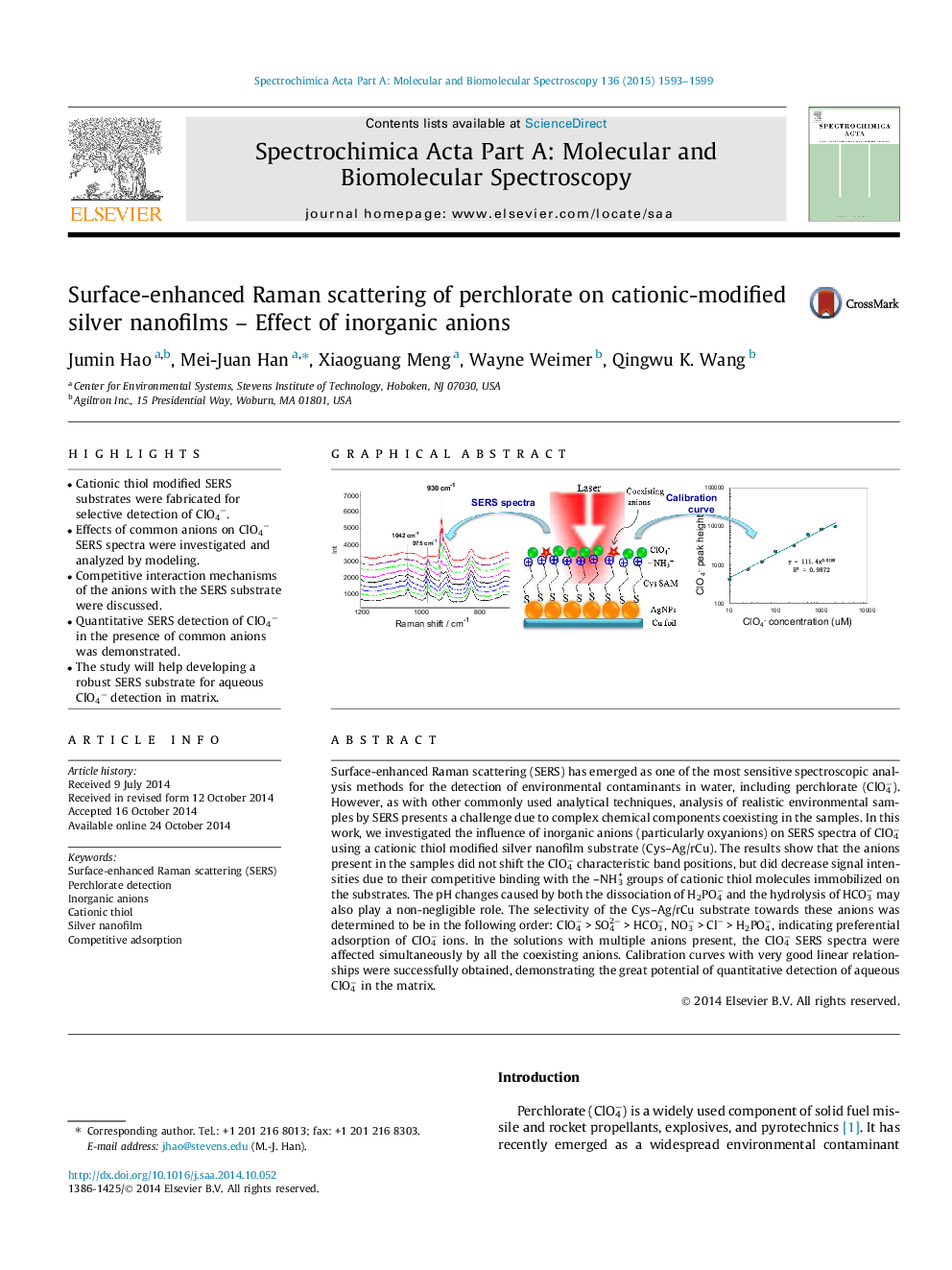
•Cationic thiol modified SERS substrates were fabricated for selective detection of ClO4−.•Effects of common anions on ClO4− SERS spectra were investigated and analyzed by modeling.•Competitive interaction mechanisms of the anions with the SERS substrate were discussed.•Quantitative SERS detection of ClO4− in the presence of common anions was demonstrated.•The study will help developing a robust SERS substrate for aqueous ClO4− detection in matrix.
Surface-enhanced Raman scattering (SERS) has emerged as one of the most sensitive spectroscopic analysis methods for the detection of environmental contaminants in water, including perchlorate (ClO4−). However, as with other commonly used analytical techniques, analysis of realistic environmental samples by SERS presents a challenge due to complex chemical components coexisting in the samples. In this work, we investigated the influence of inorganic anions (particularly oxyanions) on SERS spectra of ClO4− using a cationic thiol modified silver nanofilm substrate (Cys–Ag/rCu). The results show that the anions present in the samples did not shift the ClO4− characteristic band positions, but did decrease signal intensities due to their competitive binding with the –NH3+ groups of cationic thiol molecules immobilized on the substrates. The pH changes caused by both the dissociation of H2PO4− and the hydrolysis of HCO3− may also play a non-negligible role. The selectivity of the Cys–Ag/rCu substrate towards these anions was determined to be in the following order: ClO4− > SO42− > HCO3−, NO3− > Cl− > H2PO4−, indicating preferential adsorption of ClO4− ions. In the solutions with multiple anions present, the ClO4− SERS spectra were affected simultaneously by all the coexisting anions. Calibration curves with very good linear relationships were successfully obtained, demonstrating the great potential of quantitative detection of aqueous ClO4− in the matrix.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide