| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232471 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
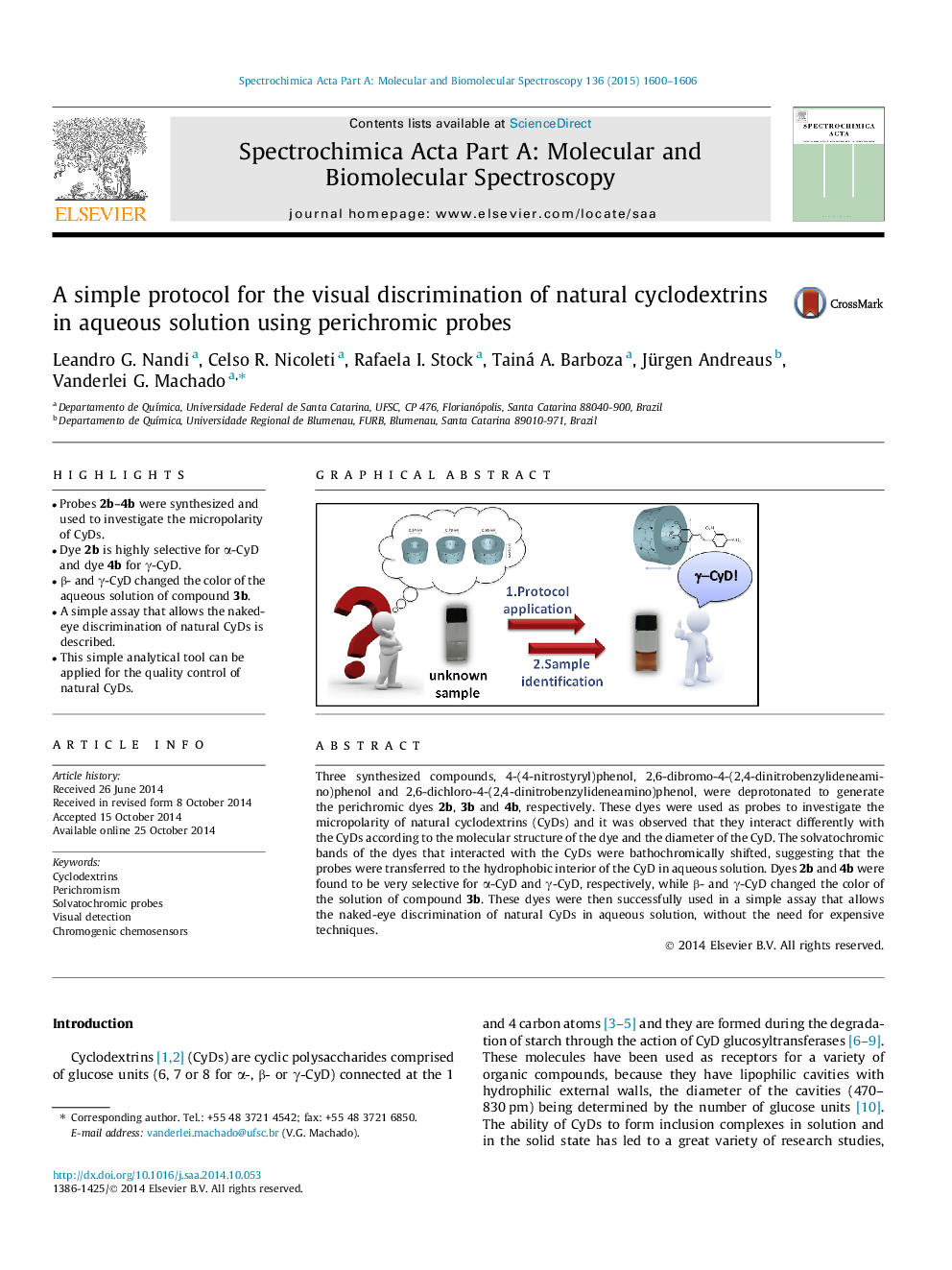
•Probes 2b–4b were synthesized and used to investigate the micropolarity of CyDs.•Dye 2b is highly selective for α-CyD and dye 4b for γ-CyD.•β- and γ-CyD changed the color of the aqueous solution of compound 3b.•A simple assay that allows the naked-eye discrimination of natural CyDs is described.•This simple analytical tool can be applied for the quality control of natural CyDs.
Three synthesized compounds, 4-(4-nitrostyryl)phenol, 2,6-dibromo-4-(2,4-dinitrobenzylideneamino)phenol and 2,6-dichloro-4-(2,4-dinitrobenzylideneamino)phenol, were deprotonated to generate the perichromic dyes 2b, 3b and 4b, respectively. These dyes were used as probes to investigate the micropolarity of natural cyclodextrins (CyDs) and it was observed that they interact differently with the CyDs according to the molecular structure of the dye and the diameter of the CyD. The solvatochromic bands of the dyes that interacted with the CyDs were bathochromically shifted, suggesting that the probes were transferred to the hydrophobic interior of the CyD in aqueous solution. Dyes 2b and 4b were found to be very selective for α-CyD and γ-CyD, respectively, while β- and γ-CyD changed the color of the solution of compound 3b. These dyes were then successfully used in a simple assay that allows the naked-eye discrimination of natural CyDs in aqueous solution, without the need for expensive techniques.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide