| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232478 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
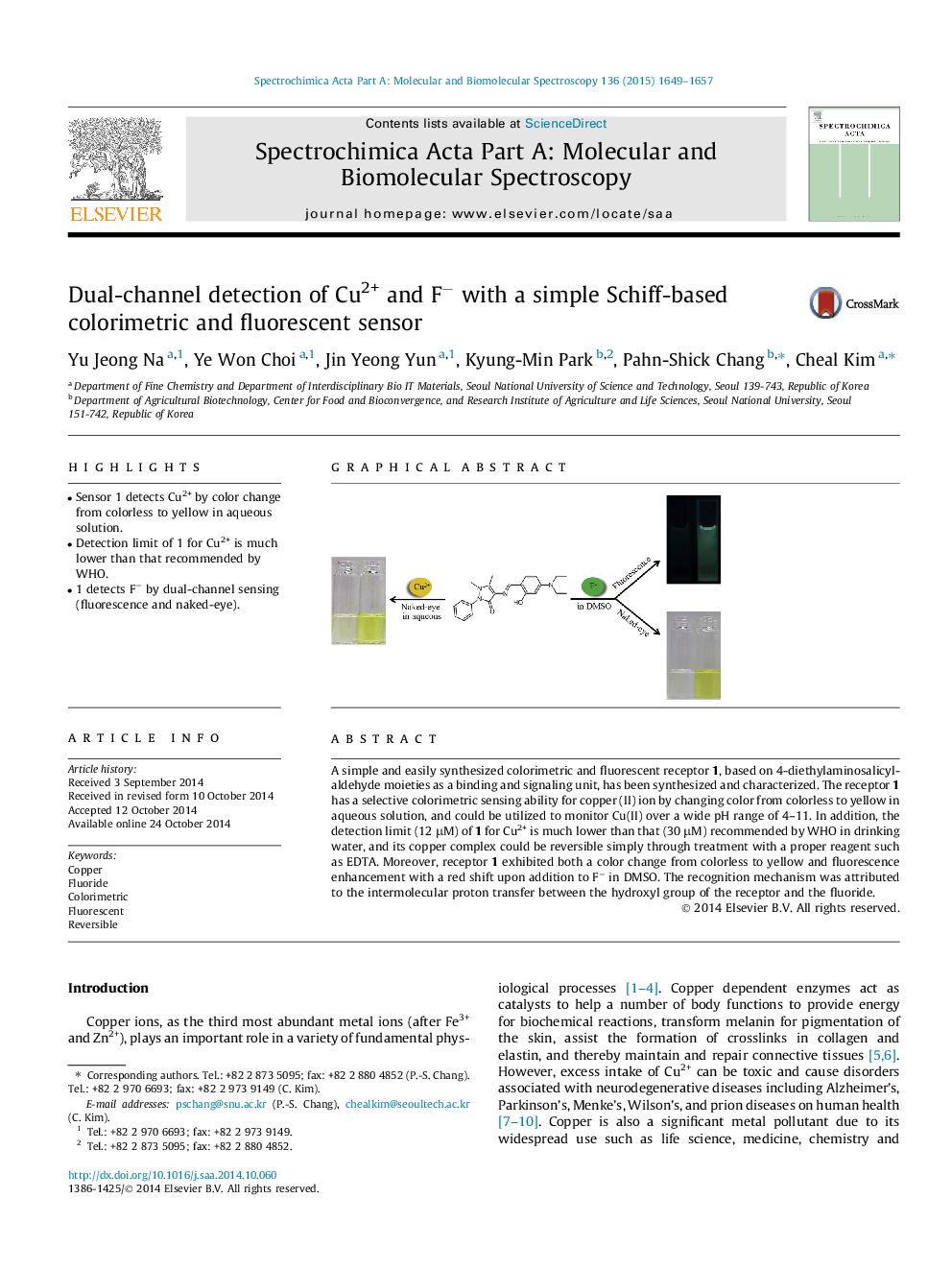
•Sensor 1 detects Cu2+ by color change from colorless to yellow in aqueous solution.•Detection limit of 1 for Cu2+ is much lower than that recommended by WHO.•1 detects F− by dual-channel sensing (fluorescence and naked-eye).
A simple and easily synthesized colorimetric and fluorescent receptor 1, based on 4-diethylaminosalicylaldehyde moieties as a binding and signaling unit, has been synthesized and characterized. The receptor 1 has a selective colorimetric sensing ability for copper (II) ion by changing color from colorless to yellow in aqueous solution, and could be utilized to monitor Cu(II) over a wide pH range of 4–11. In addition, the detection limit (12 μM) of 1 for Cu2+ is much lower than that (30 μM) recommended by WHO in drinking water, and its copper complex could be reversible simply through treatment with a proper reagent such as EDTA. Moreover, receptor 1 exhibited both a color change from colorless to yellow and fluorescence enhancement with a red shift upon addition to F− in DMSO. The recognition mechanism was attributed to the intermolecular proton transfer between the hydroxyl group of the receptor and the fluoride.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide