| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232479 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
•Simple Schiff base colorimetric sensor for Mn2+ and Fe3+ ions.•Role of substitutional group on colorimetric detection of Mn2+/Fe3+.•Sensing of Mn2+/Fe3+ using PVA chemosensor composite film.•Fluorescence sensing of Zn2+ ions and substitutional group dependent selectivity.
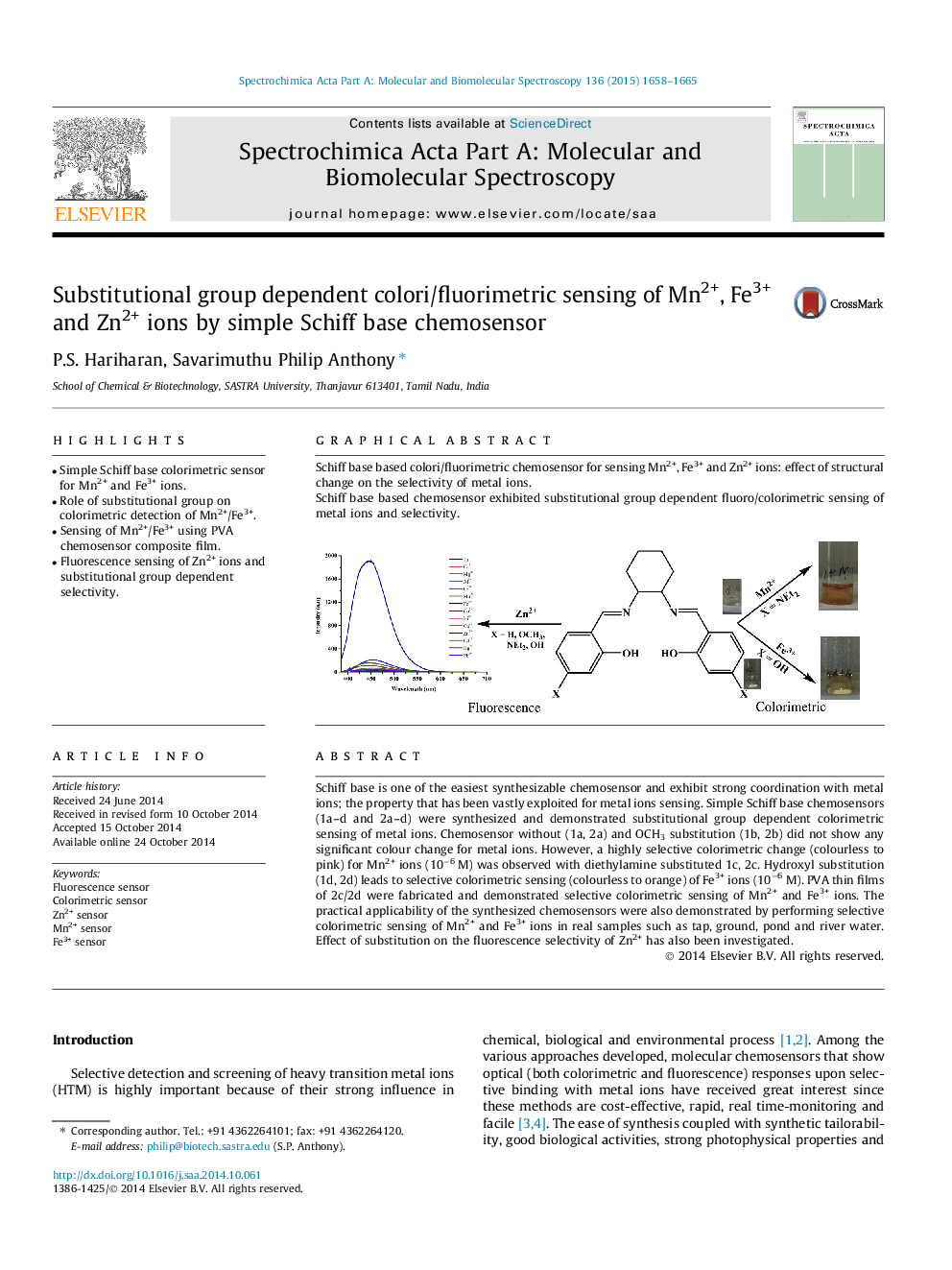
Schiff base is one of the easiest synthesizable chemosensor and exhibit strong coordination with metal ions; the property that has been vastly exploited for metal ions sensing. Simple Schiff base chemosensors (1a–d and 2a–d) were synthesized and demonstrated substitutional group dependent colorimetric sensing of metal ions. Chemosensor without (1a, 2a) and OCH3 substitution (1b, 2b) did not show any significant colour change for metal ions. However, a highly selective colorimetric change (colourless to pink) for Mn2+ ions (10−6 M) was observed with diethylamine substituted 1c, 2c. Hydroxyl substitution (1d, 2d) leads to selective colorimetric sensing (colourless to orange) of Fe3+ ions (10−6 M). PVA thin films of 2c/2d were fabricated and demonstrated selective colorimetric sensing of Mn2+ and Fe3+ ions. The practical applicability of the synthesized chemosensors were also demonstrated by performing selective colorimetric sensing of Mn2+ and Fe3+ ions in real samples such as tap, ground, pond and river water. Effect of substitution on the fluorescence selectivity of Zn2+ has also been investigated.
Graphical abstractSchiff base based colori/fluorimetric chemosensor for sensing Mn2+, Fe3+ and Zn2+ ions: effect of structural change on the selectivity of metal ions.Schiff base based chemosensor exhibited substitutional group dependent fluoro/colorimetric sensing of metal ions and selectivity.Figure optionsDownload full-size imageDownload as PowerPoint slide