| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232493 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
•Raman spectra of phenol at different pressures and temperatures were measured.•Fermi coupling coefficients were calculated based on the FR theory.•Fermi coupling coefficient turnover between 1.912 and 2.244 GPa was observed.•Fermi coupling coefficient exhibited monotonic reduction as the temperature decreased.•A conformation evolving induced by pressure and temperature on FR was analyzed.
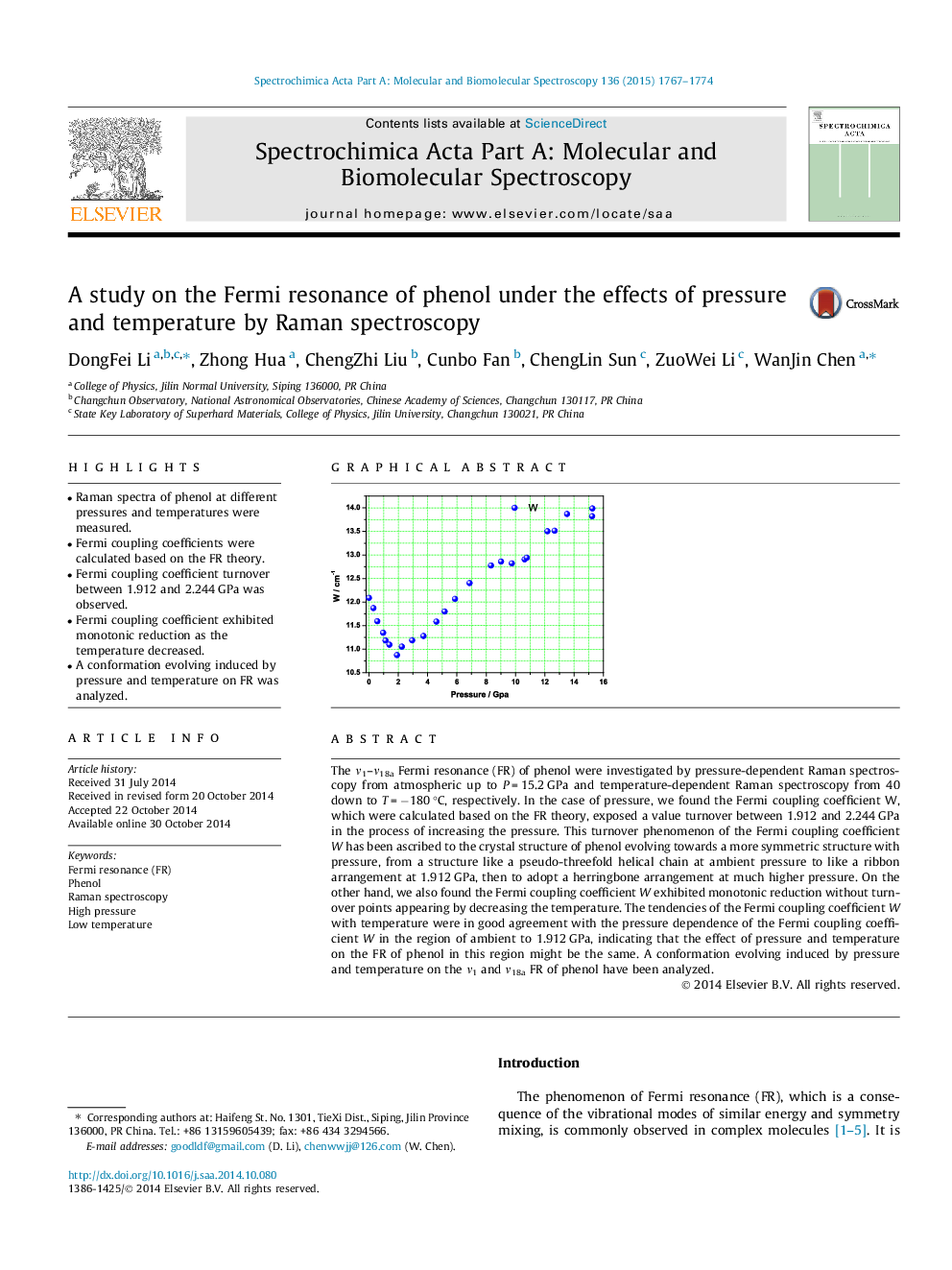
The ν1–ν18a Fermi resonance (FR) of phenol were investigated by pressure-dependent Raman spectroscopy from atmospheric up to P = 15.2 GPa and temperature-dependent Raman spectroscopy from 40 down to T = −180 °C, respectively. In the case of pressure, we found the Fermi coupling coefficient W, which were calculated based on the FR theory, exposed a value turnover between 1.912 and 2.244 GPa in the process of increasing the pressure. This turnover phenomenon of the Fermi coupling coefficient W has been ascribed to the crystal structure of phenol evolving towards a more symmetric structure with pressure, from a structure like a pseudo-threefold helical chain at ambient pressure to like a ribbon arrangement at 1.912 GPa, then to adopt a herringbone arrangement at much higher pressure. On the other hand, we also found the Fermi coupling coefficient W exhibited monotonic reduction without turnover points appearing by decreasing the temperature. The tendencies of the Fermi coupling coefficient W with temperature were in good agreement with the pressure dependence of the Fermi coupling coefficient W in the region of ambient to 1.912 GPa, indicating that the effect of pressure and temperature on the FR of phenol in this region might be the same. A conformation evolving induced by pressure and temperature on the ν1 and ν18a FR of phenol have been analyzed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide